Issn 2249-3905)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

LIFELINES of INDIA's CIVILISATION in India, a River Is a Mini-Cosmos In
LIFELINES OF INDIA’S CIVILISATION In India, a river is a mini-cosmos in concept. Every river is a mother deity who spawns mythology, art, dance, music, architecture, history and spirituality. Each one has a clear identity, appearance, value, style and spirit just like a beautiful woman. In every age, diverse human communities have reinvented themselves on river-banks with fascinating nuances…. ‘Her shimmering gold-and-white garments dazzle like a thousand suns. The jewels in her crown shine like the crescent moon. Her smiling face lights up the whole world. In her hands, she carries a pot of nectar, a symbol of immortality. Her lotus-fresh presence brings a sense of purity and joy to all beings….’. At first glance, this reads like an over-the-top flowery description of a beautiful woman coined by some besotted lover. But to those conversant with the fascinating river-lore of India, this is the mythical portrayal of the River Ganga, written by Sage Valmiki, author of India’s immortal epic Ramayan. It describes the celestial Ganga as she descends from the heavens to the earth to bring salvation to mankind. This story, known asGangavataran, is such a fundamental tenet of Indian culture that it has held countless generations of Indians in awe for millenniums. The Ganga, arguably the most picturised and written-about river in the world, has been called the Mother of India’s Spirituality and has been immortalized in sculpture, art, literature, poetry, music and dance. Following her descent to the mortal world to sanctify human efforts to attain salvation, the Ganga is perceived as mokshdayini, the Mother Goddess whose waters bring relief from sin, sorrow and suffering. -

Final Text Inside Mumbai 41 Printer Ok
B H U J AUCTIONEER OF COINS, BANK NOTES, STAMPS AND MEDALS B H U J B H U J Vimal Printery 98207 58491 98207 Printery Vimal • Mumbai Auction # 41 • Saturday, April 20, 2013 • 2:30 pm www.oswalauctions.com | [email protected] CONDITIONS OF SALE the right in forming his opinion, to consult and rely upon any expert or authority considered by him reliable. Notwithstanding the 1. Statutory Note: Antiques over 100 years old cannot be taken out forgoing, if any lot should have been wrongly described in any of India without the permission of the Director General, material aspect, the buyer may reject the same, provided that he Archaeological Survey of India, Janpath, New Delhi 110011. shall have given notice of intention to do so and shall have returned the same to the auctioneer within seven (7) days from the day of the 2. Oswal Antiques offer Life-Time Guarantee of genuineness of sale. Under no circumstances will the auctioneer be liable for any items sold. costs, expenses or damages incurred by the buyer in respect of 3. Delivery against payments, any lot, including, but not limited to, loss of any kind of profit 4. Payments can be made by Cash, Cheque or Demand Draft whatsoever. Bidders are advised to scrutinize the lots they bid (favouring OSWAL ANTIQUES payable at Mumbai). Money can be for. deposited directly in our ICICI A/c-003205004383, Dadar, 14. The auctioneer acts solely as an agent and shall not be responsible Mumbai Branch. (Intimate us payment detail by phone, SMS or for any default by either the buyer or the seller or for any action or email about the same). -
Sand Mining Violence in India in 2019-2020
Sand Mining Violence in India in 2019-2020: Detailed Compilation The illegal, excessive sand mining activities have been impacting river ecosystem and riparian communities adversely. Scores of villagers, young kids, reporters, activists and government officials are being attacked and killed year after year for objecting to and for taking action against illegal sand mining. The brazen mining is also leading to accidents which is again resulting in grievous injuries & even deaths. The situation has only deteriorated further despite several protests by locals and court orders reprimanding the Central & state governments. Moreover, given the situation where all major political parties and politicians at state level are indulgencing in unsustainable plundering of natural resource, the situation is bound to aggravate in coming years. In its 2018 overview, SANDRP could compile reports about 28 human fatalities caused by illegal and unsustainable sand mining operations across the country. The actual death toll could be much higher. Making more efforts, here SANDRP provides account of violent incidents caused by illegal & excessive sand mining happening from Jan 2019 to Nov 15, 2020. NORTH INDIA The North India covers the state of Uttar Pradesh, Haryana, Punjab, Uttarakhand and Himachal Pradesh. There is lack of media report on the issue from Jammu & Kashmir, Ladakh, Chandigarh and Delhi. Uttar Pradesh District Month River Detail Remark Year Saharanpur May Man died after hit by sand truck A truck loaded with 1 killed in 2018 mining material crushed a bike rider to death on May road 21 along the Doon Highway. The person identified as accident. Monis aged 25 died on the spot. -

Legal Instruments on Rivers in India Vol II
FOR OFFICE USE ONLY AWARDS OF INTER-STATE WATER DISPUTES TRIBUNALS CENTRAL WATER COMMISSION INTER STATE MATTERS DIRECTORATE-1 NEW DELHI JANUARY, 2018 PREFACE India has been endowed with considerable water resources through numerous small and large rivers. Some of the larger Indian Rivers like the Indus or the Ganga- Brahmaputra-Meghna are international rivers. These and most of the other rivers are the inter-State rivers. Of the total geographical area of India, approximately 95% of the area is under international or inter-State rivers. The water resources development of these rivers takes place within the legal framework of development of the inter-State rivers. A sufficient familiarity with this legal framework (that is both its generalities and the specifics of a particular problem) is therefore, an essential pre-requisite for anyone interested in Planning, Development, Operation and Management of water resources of these rivers. The basic legal instruments which need to be referred to in this context can be classified as:- 1. The Constitutional provisions relevant to inter-State rivers. 2. Treaties or agreements between India and other countries in regard to development of international rivers/ basins. 3. The Laws enacted by the Parliament in connection with the development, use and regulation of inter-State rivers. 4. The awards and the proceedings of the inter-State water disputes tribunals set up by the Central Government. 5. Notifications, Resolutions, Orders etc. issued by the Central Government in pursuance of the Laws or Tribunal awards, setting up agencies, machineries or procedures to deal with inter-State rivers, from time to time. -

Working with the Winds of Change
Working with the Toward Strategies for Responding to the Winds of Change Risks Associated with Climate Change and other Hazards Editors Second Edition Marcus Moench and Ajaya Dixit Contributors and their Institutions: Sara Ahmed Shashikant Chopde Ajaya Dixit, Anil Pokhrel and Deeb Raj Rai S. Janakarajan Fawad Khan Institute for Social and Winrock International India Institute for Social and Environmental Transition-Nepal Madras Institute of Institute for Social and Environmental Environmental Transition -India Development Studies Transition-Pakistan Marcus Moench Daanish Mustafa Madhukar Upadhya, Kanchan Mani Dixit Shiraz A. Wajih and Amit Kumar and Sarah Opitz-Stapleton King’s College London and Madav Devkota Gorakhpur Environmental Action Group Institute for Social and Environmental Transition- Nepal Water Conservation Foundation International Working with the Winds of Change Toward Strategies for Responding to the Risks Associated with Climate Change and other Hazards Second Edition Editors Marcus Moench and Ajaya Dixit Contributors and their Institutions: Sara Ahmed Shashikant Chopde Ajaya Dixit, Anil Pokhrel and Deeb Raj Rai S. Janakarajan Fawad Khan Institute for Social and Winrock International India Institute for Social and Environmental Transition-Nepal Madras Institute of Institute for Social and Environmental Environmental Transition -India Development Studies Transition-Pakistan Marcus Moench Daanish Mustafa Madhukar Upadhya, Kanchan Mani Dixit Shiraz A. Wajih and Amit Kumar and Sarah Opitz-Stapleton King’s College London and Madav Devkota Gorakhpur Environmental Action Group Institute for Social and Environmental Transition- Nepal Water Conservation Foundation International © Copyright, 2007 ProVention Consortium; Institute for Social and Environmental Transition-International; Institute for Social and Environmental Transition-Nepal This publication is made possible by the support of the ProVention Consortium. -

BIHAR FLOODS : 2007 (A Field Report)
BIHAR FLOODS : 2007 (A Field Report) Towards a disaster free India NATIONAL INSTITUTE OF DISASTER MANAGEMENT (Ministry of Home Affairs, Government of India) 5B, IIPA Campus, IP Estate, Mahatma Gandhi Marg, New Delhi-110002 (India) T: +91 11 23702432, 23705583, 23766146, F: +91 11 23702442, 23702446 I: www.nidm.gov.in BIHAR FLOODS : 2007 (A Field Report) Authors : Santosh Kumar Arun Sahdeo Sushma Guleria Bihar Floods : 2007 (A Field Report) ISBN : 978-93-82571-04-9 © NIDM, New Delhi Printing Committee : Dr. Surya Parkash Mr. Ramesh Kumar Mr. Shekhar Chaturvedi Mr. S.K. Tiwari Edition : First, 2013 Published by : National Institute of Disaster Management, Ministry of Home Affairs, New Delhi - 110002 Design and Printing: DELUXE PRINTERY, B-217, Naraina Industrial Area, Pahse-I, New Delhi-110028. Ph.: 011-45040279, 9811295120/110 PREFACE In recent past flooding and the associated risks have been rising with increased fre- quency in India which could be attributed to a number of factors, including changes in rainfall pattern, increased frequency of extreme events, in land use changes and development into flood- prone areas as a result of socio-economic demand. Of late, it has been realized that climate changes is causing significant impact on the hydrological system and increasing the risk and vulnerability to flooding. Human lives, property, environment and socio-economic are at increas- ing risk due to flooding. Bihar is highly vulnerable to floods on account of its geo-climatic conditions and various other attributing factors. The State is the most flood prone in the country in terms of percentage of land susceptible to flooding. -

MINUTES of the 55Th MEETING of the RE-CONSTITUTED EXPERT APPRAISAL COMMITTEE (EAC) on ENVIRONMENTAL IMPACT ASSESSMENT (EIA) of THERMAL POWER & COAL MINING PROJECTS
MINUTES OF THE 55th MEETING OF THE RE-CONSTITUTED EXPERT APPRAISAL COMMITTEE (EAC) ON ENVIRONMENTAL IMPACT ASSESSMENT (EIA) OF THERMAL POWER & COAL MINING PROJECTS The 55th Meeting of the reconstituted EAC (Thermal Power) was held on 5th & 6th May, 2016 in the Ministry of Environment, Forest & Climate Change at Teesta Meeting Hall, First Floor, Vayu Wing, Indira Paryavaran Bhawan, Jorbagh Road, New Delhi. The following members were present: 1. Shri Anil Kumar - Chairman 2. Prof. C.R. Babu - Member 3. Shri T.K. Dhar - Member 4. Shri N.K. Verma - Member 5. Shri J.L. Mehta - Member 6. Shri G.S. Dang - Member 7. Dr. S.D. Attri - Member (Representative of IMD) 8. Shri P.D. Siwal & N.S. Mondal - Member (Representative of CEA) 9. Dr. S.K. Paliwal - Member (Representative of CPCB) 10. Shri Manoj K Gangeya - Member Secretary Shri A.K. Bansal, Shri Shantanu Dixit, Dr. Ratnavel and Representative of WII could not be present. At the outset, the Committee welcomed Dr. Paliwal, the new representative of CPCB. Item No.1: CONFIRMATION OF THE MINUTES OF THE 54th EAC (LAST) MEETING. The Minutes of the 54th EAC (Thermal Power) meeting held on 31st March, 2016 were confirmed. Item No. 2: CONSIDERATION OF PROJECTS 2.1 4,000 MW Ultra Mega Power Project (UMPP) near Village Kanwara, Taluk Katoriya, District Banka, Bihar by M/s Bihar Mega Power Limited (A wholly owned subsidiary of PFC Ltd.) - reg. ToR (2.1.1) The Project Proponent (PP) along with their environmental Consultant, Desein Private Limited, New Delhi, made a presentation and inter-alia, provided the following information: i. -
Annual Report 2015-16
Government of India Ministry of Water Resources, River Development &Ganga Rejuvenation Ganga Flood Control Commission, Patna ANNUAL REPORT 2015-16 Kosi Barrage Table of Contents From Chairman’s Desk (ii) Highlights of the year 2015-16 (iii) 1. Introduction 1 2. Comprehensive Plans for Flood Management 15 3. Assessment of Adequacy of Waterways Under Road and Rail Bridges 17 4. Flood Management Programme 19 5. Appraisal of Flood Management and Anti-Erosion Schemes 23 6. Monitoring of Ongoing Flood Management and Anti-Erosion Schemes 33 7. River Management Activities and Works related to Border Areas 37 8. Cooperation with Neighboring Countries 46 9. Promotion of use of Hindi 53 10. Participation in Trainings, Workshops and Seminars 55 11. Representation of Ganga Flood Control Commission in Various Committees 56 Highlights of the Year 2015-16 1. Techno-economic appraisal of 63 Flood Management schemes of various Ganga Basin States was carried (including 33 schemes carried forward from previous years), out of which 22 schemes were techno-economically cleared by GFCC/AC- MoWR and 8 schemes were dropped by GFCC, whereas 33 schemes were examined and comments / observations were conveyed to the concerned State Governments. 2. Inspection/Monitoring of various Flood Management/Anti Erosion Schemes under FMP pertaining to Ganga Basin States was carried out and Central Assistance recommended. 3. GFCC monitors the progress of construction of embankments on Kamla, Bagmati and Lalbakeya rivers in Nepal being funded by Government of India. For this purpose, meetings of India-Nepal Joint Committee on Inundation & Flood Management (JCIFM) and Indian-Nepal Joint Committee on Kosi&Gandak Projects (JCKGP) were held at Kathmandu, Nepal and Patna in December, 2015 and February 2016 respectively. -

Summary Recommendations of Comprehensive Master Plans May 2015
Government of India Ministry of Water Resources, River Development & Ganga Rejuvenation Ganga Flood Control Commission FOR OFFICIAL USE Summary Recommendations of Comprehensive Master Plans May 2015 GANGA BASIN Table of Content Sl.No Comprehensive Master Plan Page 1 Main Ganga Stem River System (2004) 1 2 Adhwara River System (1988) 2 3 Ajoy River System (2002) 3 4 Badua-Chandan River System (2003) 4 5 Bagmati River System (1991) 4 6 Burhi Gandak River (2008) 5 7 Damodar River System (1996) 6 8 Gandak River System (2004 6 9 Ghaghra River System (2001) 8 10 Gomati River (1996) 8 11 Jalangi River System (1999) 9 12 Kamla-Balan River System (2005) 10 13 Kiul-Harohar River System (1993) 11 14 Kosi River System (1986) 12 15 Mahananda River System (1992) 12 16 Mayurakshi River System (1999) 13 17 Punpun River System (1997) 14 18 Ramganga River System (1999) 15 19 Rupnarayan-Haldi-Rasulpur Rivers System (2003) 15 20 Sone River System (2004) 16 21 Tons River System (2000) 17 22 Yamuna River System (2002) 18 23 Tidal River System (2003) 19 i PREFACE Two holy rivers originating from the glacial peaks of the Himalayas at an altitude of about 7000 meter, the Alaknanda and the Bhagirathi unite near Devprayag and form river Ganga which traverses its course of 2525 Km (1450 Km in Uttarakhand and Uttar Pradesh, 110 Km along Uttar Pradesh-Bihar border, 445 km in Bihar and Jharkhand and 520 km in West Bengal) before its outfall into the Bay of Bengal. Ganga basin is spread over 11 States namely, (i) Uttarakhand, (ii) Himachal Pradesh,(iii) Haryana, (iv) Delhi, (v) Uttar Pradesh, (vi) Rajasthan, (vii) Madhya Pradesh, (viii) Chhattisgarh, (ix) Bihar, (x) Jharkhand and (xi) West Bengal. -
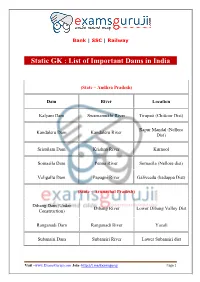
Static GK : List of Important Dams in India
Bank | SSC | Railway Static GK : List of Important Dams in India (State – Andhra Pradesh) Dam River Location Kalyani Dam Swarnamukhi River Tirupati (Chittoor Dist) Rapur Mandal (Nellore Kandaleru Dam Kandaleru River Dist) Srisailam Dam Krishna River Kurnool Somasila Dam Penna River Somasila (Nellore dist) Veligallu Dam Papagni River Galiveedu (kadappa Dist) (State – Arunachal Pradesh) Dibang Dam (Under Dibang River Lower Dibang Valley Dist Construction) Ranganadi Dam Ranganadi River Yazali Subansiri Dam Subansiri River Lower Subansiri dist Visit –www.ExamsGuruji.com Join- http://t.me/Examsguruji Page 1 Bank | SSC | Railway (State – Bihar) Chandan Dam Chandan River Banka Dist Durgawati Dam Durgawati River Bhabhua (Kaimur Dist) Nagi Dam Nagi River Jamui Dist (State – Chhattisgarh) Gangrel Dam Mahanadi River Dhamtari Dist Kutaghat Dam Kharang River Bilaspur Dist Minimata (Hasdeo) Bango Hasdeo River Korba Dist Dam Dudhawa Dam Mahanadi River Dhamtari Dist (State – Gujarat) Dantiwada Dam Banas River Banaskantha Dist Kandana Dam Mahi River Panchmahal Dist Gir National Park Kamleshwar Dam Hiran River (Junagadh Dist) Visit –www.ExamsGuruji.com Join- http://t.me/Examsguruji Page 2 Bank | SSC | Railway Sardar Sarovar Dam Narmada River Narmadha Valley Ukai Dam Tapti River Surat Dist (State – Punjab) Bakhra Nangal Dam Sutlej River Rupnagar Damsal Dam Damsal River Hoshiarpur Ranjit Sagar (Thein) Dam Ravi River Gurdaspur Siswan Dam Siswan Khad Ropar (State – Himachal Pradesh) Bhakra Nangal Dam Sutlej River Bilaspur Chamera Dam Ravi River Dalhousie Maharana -

Report of the Planning Commission Working Group on Flood
0 Government of India Planning Commission Report of Working Group on Flood Management and Region Specific Issues for XII Plan New Delhi October, 2011 1 Foreword Severe floods in the past as well as the recent floods in many States including Andhra Pradesh, Bihar, UP, Assam, West Bengal and Odisha which caused devastation and large submergence remind us of inadequacy of flood management measures. Therefore, concerted efforts are required to make a critical review of the existing flood management measures, capabilities of managers and related guidelines and policies together with state of the art technologies. I am sure that the strategies suggested in this report of the XII Plan Working Group on Flood Management and Region Specific Issues would enable the decision makers and the Planning Commission in adopting appropriate policies and measures required for flood management and coastal protection in our country. I place on record the valuable suggestions made by various members and invitees of the Working Group especially Shri R.C. Jha, the then Member(RM) & Co-Chairperson of the Working Group, Shri S.P. Kakran, Member (RM) & Co-Chairman of the Working Group. I also appreciate the outstanding efforts made by Shri Devender Sharma, Commissioner (Ganga), MOWR & Member-Secretary, Shri K.N. Keshri, Chief Engineer(FM), CWC, Shri C. Lal, Director(FMP), CWC & Nodal Officer, Shri L.K. Taneja , Sr. Joint Commissioner ( Ganga), MOWR and Shri S.L. Meena, Deputy Director(FMP), CWC and their team of other officers in drafting and giving shape to the report. October , 2011 ( Prof. Nirmal Sengupta) Chairman, IGIDR, Mumbai & Chairperson of Working Group on Flood Management & Region Specific Issues (i) Preface India has a peculiar geographical setting that there are floods in some parts and droughts in other parts and sometimes they co-exist. -
Chapter -3 Inter-State Aspects BULDHANA Man 30' 30' W-N LINK Kate Katepurna Nirguna Reservoir to Yavatmal
Chapter–3 Inter-State Aspects 3.0 General The Wainganga (Gosikhurd) - Nalganga (Purna Tapi) intra-state link project is proposed to divert surplus waters available in Wainganga river to the water short western Vidarbha for augmentation of irrigation, domestic and industrial needs in Nagpur, Wardha, Yeotmal, Amravati, Akola and Buldhana districts. The proposed diversion is planned from the existing Gosikhurd reservoir across river Wainganga located on the upstream of Pauni G&D site of CWC in Maharashtra. Wainganga, which is called Pranhita after the confluence of river Wardha, is a major tributary of river Godavari. Wainganga / Pranhita is an inter- state river with Chhattisgarh, Madhya Pradesh, Maharashtra and Telangana being the riparian States. Hence, it is necessary to look into the inter-state aspects of the link project in detail from the perspective of Godavari Water Disputes Tribunal Award and concerned inter-state Agreements. 3.1 States Traversed by the Rivers The Godavari river The Godavari is the largest river in Peninsular India and the second largest in India. It originates in Sahyadris, at an altitude of 1067 m above MSL near Triambakeshwar in Nasik district of Maharashtra and flows across Deccan plateau from Western ghats to Eastern ghats. It flows for about 1465 km in south- eastern direction through the states of Maharashtra, Telangana and Andhra Pradesh before it joins Bay of Bengal at about 96 km to the south of Rajahmundry. The Godavari basin lies between latitudes 16o - 16' N and 22o - 43' N and longitudes 73o - 26' E and 83o - 07' E. It is roughly triangular in shape.