(12) Patent Application Publication (10) Pub. No.: US 2009/0068738A1 BERTOZZ Et Al
Total Page:16
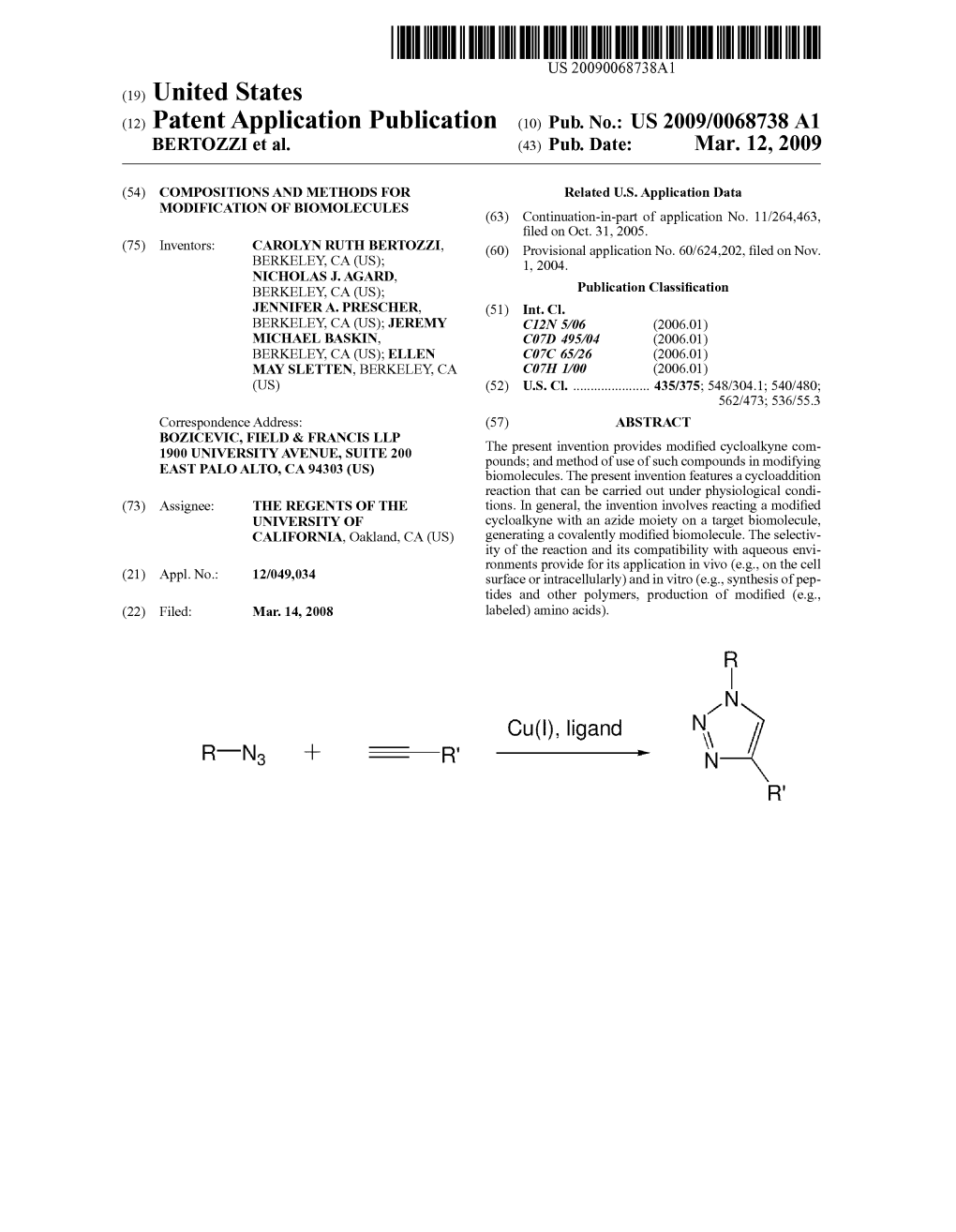
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Stabilization of Anti-Aromatic and Strained Five-Membered Rings with A
ARTICLES PUBLISHED ONLINE: 23 JUNE 2013 | DOI: 10.1038/NCHEM.1690 Stabilization of anti-aromatic and strained five-membered rings with a transition metal Congqing Zhu1, Shunhua Li1,MingLuo1, Xiaoxi Zhou1, Yufen Niu1, Minglian Lin2, Jun Zhu1,2*, Zexing Cao1,2,XinLu1,2, Tingbin Wen1, Zhaoxiong Xie1,Paulv.R.Schleyer3 and Haiping Xia1* Anti-aromatic compounds, as well as small cyclic alkynes or carbynes, are particularly challenging synthetic goals. The combination of their destabilizing features hinders attempts to prepare molecules such as pentalyne, an 8p-electron anti- aromatic bicycle with extremely high ring strain. Here we describe the facile synthesis of osmapentalyne derivatives that are thermally viable, despite containing the smallest angles observed so far at a carbyne carbon. The compounds are characterized using X-ray crystallography, and their computed energies and magnetic properties reveal aromatic character. Hence, the incorporation of the osmium centre not only reduces the ring strain of the parent pentalyne, but also converts its Hu¨ckel anti-aromaticity into Craig-type Mo¨bius aromaticity in the metallapentalynes. The concept of aromaticity is thus extended to five-membered rings containing a metal–carbon triple bond. Moreover, these metal–aromatic compounds exhibit unusual optical effects such as near-infrared photoluminescence with particularly large Stokes shifts, long lifetimes and aggregation enhancement. romaticity is a fascinating topic that has long interested Results and discussion both experimentalists and theoreticians because of its ever- Synthesis, characterization and reactivity of osmapentalynes. Aincreasing diversity1–5. The Hu¨ckel aromaticity rule6 applies Treatment of complex 1 (ref. 32) with methyl propiolate to cyclic circuits of 4n þ 2 mobile electrons, but Mo¨bius topologies (HC;CCOOCH3) at room temperature produced osmapentalyne favour 4n delocalized electron counts7–10. -

Cycloalkanes, Cycloalkenes, and Cycloalkynes
CYCLOALKANES, CYCLOALKENES, AND CYCLOALKYNES any important hydrocarbons, known as cycloalkanes, contain rings of carbon atoms linked together by single bonds. The simple cycloalkanes of formula (CH,), make up a particularly important homologous series in which the chemical properties change in a much more dramatic way with increasing n than do those of the acyclic hydrocarbons CH,(CH,),,-,H. The cyclo- alkanes with small rings (n = 3-6) are of special interest in exhibiting chemical properties intermediate between those of alkanes and alkenes. In this chapter we will show how this behavior can be explained in terms of angle strain and steric hindrance, concepts that have been introduced previously and will be used with increasing frequency as we proceed further. We also discuss the conformations of cycloalkanes, especially cyclo- hexane, in detail because of their importance to the chemistry of many kinds of naturally occurring organic compounds. Some attention also will be paid to polycyclic compounds, substances with more than one ring, and to cyclo- alkenes and cycloalkynes. 12-1 NOMENCLATURE AND PHYSICAL PROPERTIES OF CYCLOALKANES The IUPAC system for naming cycloalkanes and cycloalkenes was presented in some detail in Sections 3-2 and 3-3, and you may wish to review that ma- terial before proceeding further. Additional procedures are required for naming 446 12 Cycloalkanes, Cycloalkenes, and Cycloalkynes Table 12-1 Physical Properties of Alkanes and Cycloalkanes Density, Compounds Bp, "C Mp, "C diO,g ml-' propane cyclopropane butane cyclobutane pentane cyclopentane hexane cyclohexane heptane cycloheptane octane cyclooctane nonane cyclononane "At -40". bUnder pressure. polycyclic compounds, which have rings with common carbons, and these will be discussed later in this chapter. -

Strain-Promoted 1,3-Dipolar Cycloaddition of Cycloalkynes and Organic Azides
Top Curr Chem (Z) (2016) 374:16 DOI 10.1007/s41061-016-0016-4 REVIEW Strain-Promoted 1,3-Dipolar Cycloaddition of Cycloalkynes and Organic Azides 1 1 Jan Dommerholt • Floris P. J. T. Rutjes • Floris L. van Delft2 Received: 24 November 2015 / Accepted: 17 February 2016 / Published online: 22 March 2016 Ó The Author(s) 2016. This article is published with open access at Springerlink.com Abstract A nearly forgotten reaction discovered more than 60 years ago—the cycloaddition of a cyclic alkyne and an organic azide, leading to an aromatic triazole—enjoys a remarkable popularity. Originally discovered out of pure chemical curiosity, and dusted off early this century as an efficient and clean bio- conjugation tool, the usefulness of cyclooctyne–azide cycloaddition is now adopted in a wide range of fields of chemical science and beyond. Its ease of operation, broad solvent compatibility, 100 % atom efficiency, and the high stability of the resulting triazole product, just to name a few aspects, have catapulted this so-called strain-promoted azide–alkyne cycloaddition (SPAAC) right into the top-shelf of the toolbox of chemical biologists, material scientists, biotechnologists, medicinal chemists, and more. In this chapter, a brief historic overview of cycloalkynes is provided first, along with the main synthetic strategies to prepare cycloalkynes and their chemical reactivities. Core aspects of the strain-promoted reaction of cycloalkynes with azides are covered, as well as tools to achieve further reaction acceleration by means of modulation of cycloalkyne structure, nature of azide, and choice of solvent. Keywords Strain-promoted cycloaddition Á Cyclooctyne Á BCN Á DIBAC Á Azide This article is part of the Topical Collection ‘‘Cycloadditions in Bioorthogonal Chemistry’’; edited by Milan Vrabel, Thomas Carell & Floris P. -

WO 2013/089962 Al 20 June 2013 (20.06.2013) W P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2013/089962 Al 20 June 2013 (20.06.2013) W P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every B01J 31/04 (2006.01) B01J 31/18 (2006.01) kind of national protection available): AE, AG, AL, AM, B01J 31/14 (2006.01) B01J 31/22 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, (21) Number: International Application DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, PCT/US20 12/065285 HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, (22) International Filing Date: KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, 15 November 2012 (15.1 1.2012) ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, (25) Filing Language: English RW, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, (26) Publication Language: English TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: 13/323,328 12 December 201 1 (12. 12.201 1) US (84) Designated States (unless otherwise indicated, for every kind of regional protection available): ARIPO (BW, GH, (71) Applicant (for all designated States except US): CHEV¬ GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, RON PHILLIPS CHEMICAL COMPANY LP UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, [US/US]; 10001 Six Pines Drive, The Woodlands, Texas TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, 77380 (US). -

Highly Accelerated Inverse Electron-Demand Cycloaddition of Electron-Deficient Azides with Aliphatic Cyclooctynes
ARTICLE Received 22 Jul 2014 | Accepted 25 Sep 2014 | Published 10 Nov 2014 DOI: 10.1038/ncomms6378 Highly accelerated inverse electron-demand cycloaddition of electron-deficient azides with aliphatic cyclooctynes Jan Dommerholt1, Olivia van Rooijen2, Annika Borrmann1,Ce´lia Fonseca Guerra2, F. Matthias Bickelhaupt1,2 & Floris L. van Delft1 Strain-promoted azide–alkyne cycloaddition (SPAAC) as a conjugation tool has found broad application in material sciences, chemical biology and even in vivo use. However, despite tremendous effort, SPAAC remains fairly slow (0.2–0.5 M À 1 s À 1) and efforts to increase reaction rates by tailoring of cyclooctyne structure have suffered from a poor trade-off between cyclooctyne reactivity and stability. We here wish to report tremendous acceleration of strain-promoted cycloaddition of an aliphatic cyclooctyne (bicyclo[6.1.0]non-4-yne, BCN) with electron-deficient aryl azides, with reaction rate constants reaching 2.0–2.9 M À 1 s À 1. A remarkable difference in rate constants of aliphatic cyclooctynes versus benzoannulated cyclooctynes is noted, enabling a next level of orthogonality by a judicious choice of azide– cyclooctyne combinations, which is inter alia applied in one-pot three-component protein labelling. The pivotal role of azide electronegativity is explained by density-functional theory calculations and electronic-structure analyses, which indicates an inverse electron-demand mechanism is operative with an aliphatic cyclooctyne. 1 Institute for Molecules and Materials, Radboud University Nijmegen, Heyendaalseweg 135, 6525 AJ Nijmegen, The Netherlands. 2 Department of Theoretical Chemistry and Amsterdam Center for Multiscale Modeling, VU University Amsterdam, De Boelelaan 1083, 1081 HV Amsterdam, The Netherlands. -

Rapid Calculation of the Number of Π-Bonds, Σ-Bonds, Single and Triple Bonds in Aliphatic Unsaturated Open Chain and Cycloalkynes
World Journal of Chemical Education, 2014, Vol. 2, No. 1, 1-3 Available online at http://pubs.sciepub.com/wjce/2/1/1 © Science and Education Publishing DOI:10.12691/wjce-2-1-1 Rapid Calculation of the Number of π-bonds, σ-bonds, Single and Triple Bonds in Aliphatic Unsaturated Open Chain and Cycloalkynes Arijit Das1,*, Suman Adhikari1, Debapriya Pal2, Bijaya Paul3, R. Sanjeev4, V. Jagannadham5 1Department of Chemistry, Govt. Degree College, Dharmanagar, Tripura(N), India 2Department of Science, Netaji Vidyapith Institute, Unakoti, Tripura, India 3Department of Chemistry, Tripura University, Suryamaninagar, Tripura(W), India 4Department of Chemistry, Avanthi Degree and PG College, Hyderabad, India 5Department of Chemistry, Osmania University, Hyderabad, India *Corresponding author: [email protected] Received September 20, 2013; Revised December 15, 2013; Accepted December 30, 2013 Abstract Prediction of number of π-bonds, σ-bonds, single and triple bonds of aliphatic unsaturated open chain and cycloalkynes is a vitally important tool for students of chemistry at undergraduate and graduate level for solving different kinds of problems regarding different chemical reactions. In this manuscript, we try to present a simple and innovative method for easy calculation of number of π-bonds, σ-bonds, single and triple bonds with the help of completely 8 (eight) new formulae. Keywords: open chain and cycloalkynes, π and σ-bond, triple and single bond, number of carbon, hydrogen atoms Cite This Article: Arijit Das, Suman Adhikari, Debapriya Pal, Bijaya Paul, R. Sanjeev, and V. Jagannadham, “Rapid Calculation of the Number of π-bonds, σ-bonds, Single and Triple Bonds in Aliphatic Unsaturated Open Chain and Cycloalkynes.” World Journal of Chemical Education 2, no. -

IUPAC Nomenclature Rule for Alkyne
IUPAC nomenclature Rule for Alkyne (1) Select the longest carbon chain containing a triple bond (2) The numbering of the carbon chain is in such a way that the carbon attached to the triple bond should get the lowest possible number (3) The numbering of the triple bond carbon should be mentioned in the nomenclature (4) All other rules of substituents are the same as alkanes and alkenes ethyne 1-propyne 5-methyl-2-hexyne Nomenclature of cycloalkynes: Nomenclature is according to the suffix = cycloalkyne 4-methyl cyclopentyne cyclohexyne 3-methyl cyclobutyne IUPAC nomenclature Rule for Compounds containing both double and triple bonds (1) If the organic compound contains both double and triple bond, then select the longest carbon chain in such a way that the sum of the carbons attached to the double and triple bond should get the lowest possible number. 1-hexene-3-yne 1 + 3 = 4 (Correct numbering - small sum total) 3 + 5 = 8 (Incorrect numbering - large sum total) (2) Nomenclature is always according to the alkene followed by alkyne i.e. Prefix = alkene, Suffix = alkyne (3) If the sum of the carbons attached to double and triple bond is the same (for both LHS to RHS and RHS to LHS), then select the longest carbon chain in such a way that carbon attached to the double bond should get the lowest possible number 1-butene-3-yne 1 + 3 = 4 (Correct numbering - small sum total) 1 + 3 = 4 (Correct numbering - small sum total) (4) Numbering from LHS to RHS is correct because double bond carbon gets the lower possible number . -

Certified by
Estimation Method for the Thermochemical Properties of Polycyclic Aromatic Molecules by Joanna Yu Bachelor of Science in Engineering, Escola Politcnica-USP, Sao Paulo, Brazil, 2001 Submitted to the Department of Chemical Engineering in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Chemical Engineering Practice at the MASSACHUSETTS INSTITUTE OF TECHNOLOGY September 2004 © Massachusetts Institute of Technology 2004. All rights reserved. Author. · epartment of Chemical Engineering September 1, 2004 Certifiedbyby ............... .............. ,f.\.. ................................... (U)William H. Green, Jr. Texaco-Mangelsdorf Associate Professor Thesis Supervisor Acceptedby............ ................... ..... Daniel Blankschtein Professor of Chemical Engineering Chairman, Committee for Graduate Students MASSACHUSETTS INSTTUTE OF TECHNOLOGY F SEP 12 2005 _ 11"C4,1~e- LIBRARIES Estimation Method for the Thermochemical Properties of Polycyclic Aromatic Molecules by Joanna Yu Bachel)or of Science in Engineering, Escola Polit6cnica-USP, So Paulo, Brazil, 2001 Submitted to tle Departmenlt of Chemical Engineering on September 1, 2004, in partial fulfillment of the requiremlents for tle degree of Doctor of Philosophy in Chemical Engineering Practice Abstract Polycy( lic aromatic molecules, icluding polycyclic aromatic hydrocarbons (PAHs) have attracted c(onsiderable attention in the Ipast few decades. They are formed dur- ing the incomipliete combustion of hydrocarbon fuels and are precursors of soot. Some PAHs are known carcilnogens, and control of their emissions is an important issue. These nlolecules uarefound in Ilany materials, including coal, fuel oils, lubricants, andl carton )l lack. They are also ilmpi)licatedi tle formation of fullerenes, one of the Ilost. chemi(( ally versatile class of molecules known. Clearly, models that p)rovide predic- tive cap:,ability for their formation and growth are highly d(esiral)le. -

Mechanisms of Metal-Mediated Cyclizations
Mechanisms of Metal-Mediated Cyclizations by Benjamin Peter Warner Submitted to the Department of Chemistry in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in Organic Chemistry at the Massachusetts Institute of Technology February 1995 © Massachusetts Institute of Technology, 1995 All Rights Reserved Signature of Author.., .... ..,. ....................... ................................................................ Department of Chemistry February 1, 1995 Certified by ........................... I....................... Stephen L. Buchwald Thesis Supervisor Accepted by.............................................v..................................................................... Dietmar Seyferth Chair, Departmental Committee on Graduate Students Sciencx- This doctoral thesis has been examined by a committee of the Department of Chemistry as follows: Professor Gregory C. Fu.............................. .... ........................................................... Chair Professor Stephen L. Buchwald .................. ;................... Thesis Supervisor Professor Christopher C. Cummins.................. ................. .. ...............................; 2 Mechanisms of Metal-Mediated Cyclizations by Benjamin Peter Warner Submitted to the Department of Chemistry in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in Organic Chemistry at the Massachusetts Institute of Technology Abstract A complex of zirconocene with two rq2-alkynyl ligands is described. This -

Versatile Naphthalimide Tetrazines for Fluorogenic Bioorthogonal Labelling† Cite This: DOI: 10.1039/D1cb00128k Ab Ab Cd E Marcus E
RSC Chemical Biology View Article Online PAPER View Journal Versatile naphthalimide tetrazines for fluorogenic bioorthogonal labelling† Cite this: DOI: 10.1039/d1cb00128k ab ab cd e Marcus E. Graziotto, Liam D. Adair, Amandeep Kaur, Pauline Ve´rite´, Sarah R. Ball,c Margaret Sunde, cd Denis Jacquemin e and Elizabeth J. New *abd Fluorescent probes for biological imaging have revealed much about the functions of biomolecules in health and disease. Fluorogenic probes, which are fluorescent only upon a bioorthogonal reaction with a specific partner, are particularly advantageous as they ensure that fluorescent signals observed in biological imaging arise solely from the intended target. In this work, we report the first series of naphthalimide tetrazines for bioorthogonal fluorogenic labelling. We establish that all of these compounds can be used for imaging through photophysical, analytical and biological studies. The best candidate was Np6mTz, where the tetrazine ring is appended to the naphthalimide at its 6-position via a Creative Commons Attribution-NonCommercial 3.0 Unported Licence. phenyl linker in a meta configuration. Taking our synthetic scaffold, we generated two targeted variants, LysoNpTz and MitoNpTz, which successfully localized within the lysosomes and mitochondria Received 10th June 2021, respectively, without the requirement of genetic modification. In addition, the naphthalimide tetrazine Accepted 24th June 2021 system was used for the no-wash imaging of insulin amyloid fibrils in vitro, providing a new method that DOI: 10.1039/d1cb00128k can monitor their growth kinetics and morphology. Since our synthetic approach is simple and modular, these new naphthalimide tetrazines provide a novel scaffold for a range of bioorthogonal tetrazine- rsc.li/rsc-chembio based imaging agents for selective staining and sensing of biomolecules. -

Max-Planck-Institut Für Kohlenforschung Report for The
Max-Planck-Institut für Kohlenforschung Report for the Period of January 2002 – December 2004 Printed May 2005 Max-Planck-Institut für Kohlenforschung Kaiser-Wilhelm-Platz 1 45470 Mülheim an der Ruhr, Germany Tel. +49 208 3 06 1 Fax +49 208 3 06 29 80 http://www.mpi-muelheim.mpg.de Managing Director Professor Dr. Ferdi Schüth Director of the Department of Synthetic Organic Chemistry Professor Dr. Manfred T. Reetz Tel. +49 208 3 06 20 00 Fax +49 208 3 06 29 85 E-mail: [email protected] Director of the Department of Homogeneous Catalysis NN Director of the Department of Heterogeneous Catalysis Professor Dr. Ferdi Schüth Tel. +49 208 3 06 23 73 Fax +49 208 3 06 29 95 E-mail: [email protected] Director of the Department of Organometallic Chemistry Professor Dr. Alois Fürstner Tel. +49 208 3 06 23 42 Fax +49 208 3 06 29 94 E-mail: [email protected] Director of the Department of Theory Professor Dr. Walter Thiel Tel. +49 208 3 06 21 50 Fax +49 208 3 06 29 96 E-mail: [email protected] External Scientific Members of the Max-Planck-Institut für Kohlenforschung Professor Dr. Alois Haas Medonstrasse 17 14532 Kleinmachnow Germany Professor Dr. Jack Halpern University of Chicago Department of Chemistry 5735 South Ellis Avenue Chicago, Illinois 60637 USA Professor Dr. Walter Leitner Lehrstuhl für Technische Chemie und Petrolchemie Institut für Technische und Makromolekulare Chemie Rheinisch-Westfälische Technische Hochschule Aachen Worringer Weg 1 52074 Aachen Germany Member of the Scientific Council of the Max Planck Society, Section of Chemistry, Physics and Technology Professor Dr. -

From an Enediyne Core Biosynthetic Hypothesis to the Hexadehydro-Diels–Alder Reaction
Spontaneity to Serendipity: From an Enediyne Core Biosynthetic Hypothesis to the Hexadehydro-Diels–Alder Reaction A DISSERTATION SUBMITTED TO THE FACULTY OF THE GRADUATE SCHOOL OF THE UNIVERSITY OF MINNESOTA BY Brian Patrick Woods IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY Thomas R. Hoye, Adviser August 2014 © Brian Patrick Woods 2014 i Acknowledgements While my name is on the first page of this thesis, countless family, friends, and colleagues contributed their knowledge, support, and love to help make this document a reality. First and foremost, I need to thank my family. I owe everything to my parents, Steve and Diane, who were my first teachers and have been and continue to be inspiring examples of how a life of constant learning and exploration never gets old. They instilled in me their value in the benefits of education and the doors it opens for those willing to knock. For both my siblings and I, they always encouraged us to pursue our dreams and desires—from CSI investigator, to Broadway actor, to NASA scientist—and provided us with the guidance and support to reach them. To my sister and brother, Shannon and Tyler, I thank them for their innate ability to keep me grounded and their unwavering confidence in me. To each of them, along with their partners Braxton and Esther, and my nephew Harrison, I thank them for being a constant reminder that there’s nothing more important than family. For their continual love I would also like to thank my extended family of aunts, uncles, and cousins—and more specifically my grandparents Patrick and Virginia Woods.