Original Contribution Are Children with Birth Defects at Higher Risk Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Advantage of Genome-Wide Microarrays Over Targeted Approaches
PDF hosted at the Radboud Repository of the Radboud University Nijmegen The following full text is a publisher's version. For additional information about this publication click this link. http://hdl.handle.net/2066/70828 Please be advised that this information was generated on 2021-09-24 and may be subject to change. COPY NUMBER VARIATION AND MENTAL RETARDATION opmaak koolen.indd 1 10-09-2008 10:11:31 Copy number variation and mental retardation The studies presented in this thesis were performed at the Department of Human Genetics, Radboud University Nijmegen Medical Center, Nijmegen, the Netherlands. The research was supported by a grant from the Netherlands Organization for Health Research and Development (ZonMw). Publication of this thesis was financially supported by the Department of Human Genetics, Radboud University Nijmegen Medical Center, Nijmegen, the Netherlands. ISBN/EAN 978-90-6464-290-6 © 2008 D.A. Koolen All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, by print or otherwise, without permission in writing from the author. Cover photo: Printed by: Ponsen & Looijen B.V., Wageningen opmaak koolen.indd 2 10-09-2008 10:11:31 Copy number variation and mental retardation Een wetenschappelijke proeve op het gebied van de Medische Wetenschappen Proefschrift ter verkrijging van de graad doctor aan de Radboud Universiteit Nijmegen op gezag van de rector magnificus prof. mr. S.C.J.J. Kortmann, volgens besluit van het College van Decanen in het openbaar te verdedigen op donderdag 6 november 2008 om 15.30 uur precies door David Aljosja Koolen geboren op 22 juni 1976 te ‘s-Gravenhage opmaak koolen.indd 3 10-09-2008 10:11:32 Promotor: Prof. -

Whole Chromosome Gain Does Not in Itself Confer Cancer-Like Chromosomal Instability
Whole chromosome gain does not in itself confer cancer-like chromosomal instability Anders Valinda,1, Yuesheng Jina, Bo Baldetorpb, and David Gisselssona aDepartment of Clinical Genetics, Lund University, University and Regional Laboratories, Biomedical Center B13, Lund SE22184, Sweden; and bDepartment of Oncology, Lund University, Skåne University Hospital, Lund SE22185, Sweden Edited* by George Klein, Karolinska Institutet, Stockholm, Sweden, and approved November 4, 2013 (received for review June 12, 2013) Constitutional aneuploidy is typically caused by a single-event and chromosomal instability in humans is using constitutional meiotic or early mitotic error. In contrast, somatic aneuploidy, aneuploidy syndromes as a model. Cells from patients with these found mainly in neoplastic tissue, is attributed to continuous syndromes provide a good experimental system for studying the chromosomal instability. More debated as a cause of aneuploidy effects of aneuploidy on overall genome stability on representative is aneuploidy itself; that is, whether aneuploidy per se causes human material. Such cells typically only have a single or a limited chromosomal instability, for example, in patients with inborn set of stem-line chromosome aberrations compared with tumor aneuploidy. We have addressed this issue by quantifying the level cell lines, which typically harbor a multitude of genetic lesions, as of somatic mosaicism, a proxy marker of chromosomal instability, well as a cancer phenotype. The few earlier studies performed on in patients with -

95 Birth Defects Exec Summ
Executive Summary In 1995, 865 cases of birth defects were detected among live born infants and fetuses of 20 or more weeks gestation delivered to mothers residing in Texas Public Health Regions 6 and 11 (Houston/Galveston region and Lower Rio Grande Valley). In this first full year of the Registry, surveillance was limited to approximately 23 major categories of birth defects, comprising 30 to 40 percent of all known structural malformations. Down syndrome, oral clefts, and spina bifida were the most common birth defects, although some major structural malformations were not yet monitored in 1995. Age Patterns: Both trisomy 21 (Down syndrome) and trisomy 18 (Edwards syndrome) demonstrated an age-specific rate pattern that was J-shaped, with the highest birth prevalence (“rates”) observed among mothers 35 years of age and older. Gastroschisis, a malformation of the abdominal wall, exhibited highest rates among the youngest mothers and decreased with each older age group. Racial/Ethnic Patterns: Anencephaly and spina bifida were lowest among African Americans; however, rates were similar in whites and Hispanics. Among the heart defects, relatively high rates of tetralogy of Fallot were observed among African Americans, although they exhibited relatively low rates of hypoplastic left heart. Cleft palate alone was more likely to occur among whites and Hispanics. Rates of trisomy 18 (Edwards syndrome) were highest among African Americans and whites. Gender Patterns: With regard to sex, higher rates were documented among females for anencephaly, and to a lesser extent, spina bifida. Females experienced two-fold higher rates of trisomy 18 (Edwards syndrome). Males had higher rates of cleft lip with or without cleft palate. -

Application of Neural Networks for Classification of Patau, Edwards
Catic et al. BMC Medical Genomics (2018) 11:19 DOI 10.1186/s12920-018-0333-2 RESEARCHARTICLE Open Access Application of Neural Networks for classification of Patau, Edwards, Down, Turner and Klinefelter Syndrome based on first trimester maternal serum screening data, ultrasonographic findings and patient demographics Aida Catic1,2* , Lejla Gurbeta1,4, Amina Kurtovic-Kozaric1,3, Senad Mehmedbasic2 and Almir Badnjevic1,4,5,6 Abstract Background: The usage of Artificial Neural Networks (ANNs) for genome-enabled classifications and establishing genome-phenotype correlations have been investigated more extensively over the past few years. The reason for this is that ANNs are good approximates of complex functions, so classification can be performed without the need for explicitly defined input-output model. This engineering tool can be applied for optimization of existing methods for disease/syndrome classification. Cytogenetic and molecular analyses are the most frequent tests used in prenatal diagnostic for the early detection of Turner, Klinefelter, Patau, Edwards and Down syndrome. These procedures can be lengthy, repetitive; and often employ invasive techniques so a robust automated method for classifying and reporting prenatal diagnostics would greatly help the clinicians with their routine work. Methods: The database consisted of data collected from 2500 pregnant woman that came to the Institute of Gynecology, Infertility and Perinatology “Mehmedbasic” for routine antenatal care between January 2000 and December 2016. During first trimester all women were subject to screening test where values of maternal serum pregnancy- associated plasma protein A (PAPP-A) and free beta human chorionic gonadotropin (β-hCG) were measured. Also, fetal nuchal translucency thickness and the presence or absence of the nasal bone was observed using ultrasound. -

SUPPORTING FAMILIES, CELEBRATING LIVES a WORD from 2 Our Chair
SUMMER 2019 Soft UK SUPPORTING FAMILIES, CELEBRATING LIVES A WORD FROM 2 Our Chair Welcome to a packed newsletter, full to participate of family stories, information and in some work updates. We hope you enjoy reading being carried and finding out what is going on in out by the University of Leeds, into the world of SOFT UK! the delivery of challenging news There is so much in these pages but after ultrasound scan. They are I would just like to highlight one focusing on identifying the specific article – the blog that Alison Pearson words, phrases and behaviours that (mum to Isabel) has written, with sonographers should use to deliver another mum of a little girl with different types of unexpected or Down’s syndrome, for Public Health challenging news that they identify England on the use of language. during ultrasound. Interestingly, there is no widespread agreement on We know that the words we speak how this should be done. The result can leave a huge impact and create is that sonographers often decide a lasting memory - either good or for themselves what works best, and bad; for those who hear them. Joel their conclusions are not always in Osteen said: "Be careful what you line with parent preferences. say. You can say something hurtful One, small step, but hopefully we in ten seconds, but ten years later, can get your views and experiences the wounds are still there." We hear across and make a difference for that from many families when they families in the future. talk of how they were told of a high Enjoy the rest of the newsletter risk or diagnosis of Trisomy, or when and do keep letting us know your medical professionals talk about the feedback, contributions and ideas for future for their child with Edwards’ or future editions. -

Double Aneuploidy in Down Syndrome
Chapter 6 Double Aneuploidy in Down Syndrome Fatma Soylemez Additional information is available at the end of the chapter http://dx.doi.org/10.5772/60438 Abstract Aneuploidy is the second most important category of chromosome mutations relat‐ ing to abnormal chromosome number. It generally arises by nondisjunction at ei‐ ther the first or second meiotic division. However, the existence of two chromosomal abnormalities involving both autosomal and sex chromosomes in the same individual is relatively a rare phenomenon. The underlying mechanism in‐ volved in the formation of double aneuploidy is not well understood. Parental ori‐ gin is studied only in a small number of cases and both nondisjunctions occurring in a single parent is an extremely rare event. This chapter reviews the characteristics of double aneuploidies in Down syndrome have been discussed in the light of the published reports. Keywords: Double aneuploidy, Down Syndrome, Klinefelter Syndrome, Chromo‐ some abnormalities 1. Introduction With the discovery in 1956 that the correct chromosome number in humans is 46, the new area of clinical cytogenetic began its rapid growth. Several major chromosomal syndromes with altered numbers of chromosomes were reported, such as Down syndrome (trisomy 21), Turner syndrome (45,X) and Klinefelter syndrome (47,XXY). Since then it has been well established that chromosome abnormalities contribute significantly to genetic disease resulting in reproductive loss, infertility, stillbirths, congenital anomalies, abnormal sexual development, mental retardation and pathogenesis of malignancy [1]. Clinical features of patients with common autosomal or sex chromosome aneuploidy is shown in Table 1. © 2015 The Author(s). Licensee InTech. This chapter is distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. -

The Combined Test
Antenatal Screening Antenatal Screening for Down's syndrome, Edwards syndrome and Patau syndrome The Combined Test Questions and Answers for women considering the test The Wolfson Institute of Preventive Medicine Barts and The London School of Medicine and Dentistry Antenatal Screening This leaflet answers some of the common questions women ask about their screening test – we hope you find it helpful. You are welcome to discuss the test with your midwife or consultant before you decide whether you would like to be screened. If you have any further questions screening staff at the Wolfson Institute are available to talk to you on 020 7882 6293. What is Down's syndrome? What are Edwards and Patau syndrome? Down's syndrome (trisomy 21) is Edwards syndrome (trisomy 18) is defined by the presence of an extra defined by the presence of an extra chromosome number 21 in the cells chromosome number 18 in the cells of the fetus or affected individual. In of the fetus or affected individual an unscreened population about 1 in while Patau syndrome (trisomy 13) every 500 babies is born with Down's is defined by an extra chromosome syndrome. Usually it is not inherited number 13 in the cells of the fetus or and so a baby can be affected affected individual. Both syndromes even if there is no history of Down's affect multiple organs with a high risk syndrome in the family. of fetal death. Down's syndrome is the most At 12 weeks of pregnancy Edwards common cause of severe learning syndrome has a prevalence of about disability and is often associated 1 in 1,500 and Patau syndrome has a with physical problems such as heart prevalence of about 1 in 3,500 in an defects and difficulties with sight and unscreened population. -

Congenital Heart Disease and Chromossomopathies Detected By
Review Article DOI: 10.1590/0103-0582201432213213 Congenital heart disease and chromossomopathies detected by the karyotype Cardiopatias congênitas e cromossomopatias detectadas por meio do cariótipo Cardiopatías congénitas y anomalías cromosómicas detectadas mediante cariotipo Patrícia Trevisan1, Rafael Fabiano M. Rosa2, Dayane Bohn Koshiyama1, Tatiana Diehl Zen1, Giorgio Adriano Paskulin1, Paulo Ricardo G. Zen1 ABSTRACT Conclusions: Despite all the progress made in recent de- cades in the field of cytogenetic, the karyotype remains an es- Objective: To review the relationship between congenital sential tool in order to evaluate patients with congenital heart heart defects and chromosomal abnormalities detected by disease. The detailed dysmorphological physical examination the karyotype. is of great importance to indicate the need of a karyotype. Data sources: Scientific articles were searched in MED- LINE database, using the descriptors “karyotype” OR Key-words: heart defects, congenital; karyotype; Down “chromosomal” OR “chromosome” AND “heart defects, syndrome; trisomy; chromosome aberrations. congenital”. The research was limited to articles published in English from 1980 on. RESUMO Data synthesis: Congenital heart disease is characterized by an etiologically heterogeneous and not well understood Objetivo: Realizar uma revisão da literatura sobre a group of lesions. Several researchers have evaluated the pres- relação das cardiopatias congênitas com anormalidades ence of chromosomal abnormalities detected by the karyo- cromossômicas detectadas por meio do exame de cariótipo. type in patients with congenital heart disease. However, Fontes de dados: Pesquisaram-se artigos científicos no most of the articles were retrospective studies developed in portal MEDLINE, utilizando-se os descritores “karyotype” Europe and only some of the studied patients had a karyo- OR “chromosomal” OR “chromosome” AND “heart defects, type exam. -

Turner's Syndrome
SYLLABUS ❑Down’s Syndrome, ❑Inborn errors of metabolism: ❑Turner’s syndrome, phenylketonuria,albinism,galactosemia ❑Kleinfelter’s syndrome, ❑Genetic counseling ❑Edwards syndrome, DOWN SYNDROME • Down syndrome (sometimes called Down’s syndrome) is a condition in which a child is born with an extra copy of their 21st chromosome —hence its other name, trisomy 21. This causes physical and mental developmental delays and disabilities DOWN SYNDROME Causes • In all cases of reproduction, both parents pass their genes on to their children. These genes are carried in chromosomes. • When the baby’s cells develop, each cell is supposed to receive 23 pairs of chromosomes, for 46 chromosomes total. Half of the chromosomes are from the mother, and half are from the father. • In children with Down syndrome, one of the chromosomes doesn’t separate properly. The baby ends up with three copies, or an extra partial copy, of chromosome 21, instead of two. This extra chromosome causes problems as the brain and physical features develop. Types Trisomy 21 • Trisomy 21 means there’s an extra copy of chromosome 21 in every cell. • This is the most common form of Down syndrome. Mosaicism • Mosaicism occurs when a child is born with an extra chromosome in some but not all of their cells. • People with mosaic Down syndrome tend to have fewer symptoms than those with trisomy 21. Translocation • In this type of Down syndrome, children have only an extra part of chromosome 21. There are 46 total chromosomes. However, one of them has an extra piece of chromosome 21 attached. risk factors • . According to the Centers for Disease and Prevention, mothers aged 35 and older are more likely to have a baby with Down syndrome than younger mothers. -

Soft UK Your Unborn Baby A5 04.10 37045
SOFT UK Support Organisation for Trisomy 13/18 and related disorders Charity number 1002918 www.soft.org.uk Your Unborn Baby SOFT provides support for families affected by Patau’s syndrome (trisomy 13), Edwards’ syndrome (trisomy 18), partial trisomy, mosaicism, rings, translocation, deletion and related abnormalities and rare conditions including holoprosencephaly. Truth has no special time of its own. Its hour is now. Always. Albert Schweitzer YOUR UNBORN BABY INDEX Section Page About Your Unborn Baby 2 SOFT UK Booklets 4 For Professionals 4 Chromosome Defects, What are They? 5 Trisomy 7 Holoprosencephaly 8 Related Disorders 9 Genetic Information 10 Screening Tests 13 Diagnostic tests 15 Telling Parents 18 Making a Decision 19 When the Pregnancy is Terminated 22 When the Pregnancy Continues 26 Caring for a Baby with a Related Disorder 32 Bereavement 34 How Brothers and Sisters are Affected 39 A New Baby 43 Acknowledgments 45 SOFT UK 45 Information for Parents 45 Other Sources of Support for Parents 47 1 ABOUT YOUR UNBORN BABY Your Unborn Baby was compiled by Rachel Attwell, Jenny Robbins, and Christine Rose, with the help of SOFT medical advisers and families affected by trisomy 13/18 who have shared their own personal experiences. Every family is unique. What may be right for one child or family is not necessarily right for another, and parents can use the shared memories in these booklets as a basis for discussion between themselves and their medical advisers. SOFT does not recommend particular methods of treatment, and new treatment must never be started or existing treatment changed without consultation with your doctor and other medical professionals. -
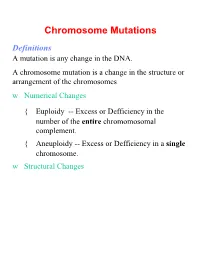
Chromosome Mutations Definitions a Mutation Is Any Change in the DNA
Chromosome Mutations Definitions A mutation is any change in the DNA. A chromosome mutation is a change in the structure or arrangement of the chromosomes w Numerical Changes { Euploidy -- Excess or Defficiency in the number of the entire chromomosomal complement. { Aneuploidy -- Excess or Defficiency in a single chromosome. w Structural Changes Numerical Chromosome Mutations Euploid -- “True Number” The cell(s) have the same number of copies of all the chromosome. 1. Monoploid (Haploid if a gamete) 2. Diploid (Often is not considered a Euploid) 3. Triploid 4. Tetraploid Euploidy Chromosomes shown at metaphase Normal Diploid Euploid Monoploid Triploid Tetraploid Euploidy is an excess or deficiency for the entire complement of chromosomes. Euploidy Organisms w Plants, amphibians, lower reptiles w Some cells in other organisms (liver, midgut, etc.) Root Causes w Nondisjunction w Autoreduplication Euploidy (continued) Origins w Autopolyploidy { Often involving individuals within the same species. { Infertile. Propagation by cloning. w Allopolyploidy { Often involving individuals from different species. { Fertile. Dosage Concerns Examples Numerical Chromosome Mutations Aneuploidy Excess or Defficiency in a single chromosome. 1. Monosomy 2. Disomy (only if normally cell is not diploid) 3. Trisomy 4. Tetrasomy Aneuploidy Chromosomes shown at metaphase Normal Diploid Aneuploid Monosomic Trisomic Tetrasomic Aneuploidy is an excess or deficiency for fewer than the entire complement of chromosomes. Aneuploidy Organisms w All organisms w Variability in how they deal with it Root Causes w Nondisjunction w Results of Chromosome Structure Mutations Aneuploidy -- Examples Plants w Jimson Weed (Datura stramonium) Animals w Drosophila: Triploid females are viable & fertile. Trisomy flies are not! w Human (autosomal): { Trisomy 13 (Patau Syndrome) 1:20,000 { Trisomy 18 (Edwards Syndrome) 1:10,000 { Trisomy 21 (Down Syndrome) 1:700 { Other rare ones (7, 9, 12, 14, & 22) Aneuploidy Examples w Human Sex Determining Chromosomes 1. -

Chromosomal Disorders: Background and Neuroradiology
Chromosomal Disorders: Background and Neuroradiology Ashok J. Kumar, 1 Thomas P. Naidich, Gail Stetten, Allan L. Reiss, Henry Wang, George H. Thomas, and OrestHurko From the Division of Neuroradiology (AJK, HW), Departments of Neurology (OH), Psychiatry (ALR), and Gynecology and Obstetrics (GS), The Johns Hopkins University School of Medicine; and the Kennedy Institutes, Baltimore, MD (GHT) and Baptist Hospital (TPN), Miami, FL Chromosomal aberrations, and their associated malities can be detected by neuroimaging. 2) syndromes of dysmorphism and malformations Most of the clinical syndromes associated with of multiple organ systems, have been long rec chromosomal abnormalities are notoriously vari ognized as significant causes of severe mental able, requiring individual assessment of each pa impairment. With a few major exceptions, these tient. Incompletely overlapping partial aneu clinical syndromes have only recently been cat ploidies, for example, give rise to broadly similar alogued as systematically ( 1, 2) as have been syndromes, but slight differences in their extent single-gene disorders. The neuropathologic fea may considerably alter the resultant phenotype. tures of many of these syndromes have been Neuroimaging can help to characterize the asso characterized only broadly, if at all. Not surpris ciated CNS pathology and facilitate proper man ingly, there is no detailed compendium of the agement of the patient. neuroradiologic features of the malformations of It is our purpose to introduce the chromosomal the central nervous system (CNS) associated with disorders to the general neuroradiologist by con various chromosomal aberrations. centrating on basic concepts and by giving spe Certain chromosomal aberrations, such as tri cific illustrations of some of the more commonly somy 21 and the associated Down syndrome, are encountered syndromes.