682.Full.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tolerance and Resistance to Organic Nitrates in Human Blood Vessels
\ö-\2- Tolerance and Resistance to Organic Nitrates in Human Blood Vessels Peter Radford Sage MBBS, FRACP Thesis submit.ted for the degree of Doctor of Philosuphy Department of Medicine University of Adelaide and Cardiology Unit The Queen Elizabeth Hospital I Table of Gontents Summary vii Declaration x Acknowledgments xi Abbreviations xil Publications xtil. l.INTRODUCTION l.L Historical Perspective I i.2 Chemical Structure and Available Preparations I 1.3 Cellular/biochemical mechanism of action 2 1.3.1 What is the pharmacologically active moiety? 3 1.3.2 How i.s the active moiety formed? i 4 1.3.3 Which enzyme system(s) is involved in nitrate bioconversi<¡n? 5 1.3.4 What is the role of sulphydryl groups in nitrate action? 9 1.3.5 Cellular mechanism of action after release of the active moiety 11 1.4 Pharmacokinetics t2 1.5 Pharmacological Effects r5 1.5.1 Vascular effects 15 l.5.2Platelet Effects t7 1.5.3 Myocardial effects 18 1.6 Clinical Efhcacy 18 1.6.1 Stable angina pectoris 18 1.6.2 Unstable angina pectoris 2t 1.6.3 Acute myocardial infarction 2l 1.6.4 Congestive Heart Failure 23 ll 1.6.5 Other 24 1.7 Relationship with the endothelium and EDRF 24 1.7.1 EDRF and the endothelium 24 1.7.2 Nitrate-endothelium interactions 2l 1.8 Factors limiting nitrate efficacy' Nitrate tolerance 28 1.8.1 Historical notes 28 1.8.2 Clinical evidence for nitrate tolerance 29 1.8.3 True/cellular nitrate tolerance 31 1.8.3.1 Previous studies 31 | .8.3.2 Postulated mechanisms of true/cellular tolerance JJ 1.8.3.2.1 The "sulphydryl depletion" hypothesis JJ 1.8.3.2.2 Desensitization of guanylate cyclase 35 1 8.i.?..3 Impaired nitrate bioconversion 36 1.8.3.2.4'Ihe "superoxide hypothesis" 38 I.8.3.2.5 Other possible mechanisms 42 1.8.4 Pseudotolerance ; 42 1.8.4. -

A Textbook of Clinical Pharmacology and Therapeutics This Page Intentionally Left Blank a Textbook of Clinical Pharmacology and Therapeutics
A Textbook of Clinical Pharmacology and Therapeutics This page intentionally left blank A Textbook of Clinical Pharmacology and Therapeutics FIFTH EDITION JAMES M RITTER MA DPHIL FRCP FMedSci FBPHARMACOLS Professor of Clinical Pharmacology at King’s College London School of Medicine, Guy’s, King’s and St Thomas’ Hospitals, London, UK LIONEL D LEWIS MA MB BCH MD FRCP Professor of Medicine, Pharmacology and Toxicology at Dartmouth Medical School and the Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire, USA TIMOTHY GK MANT BSC FFPM FRCP Senior Medical Advisor, Quintiles, Guy's Drug Research Unit, and Visiting Professor at King’s College London School of Medicine, Guy’s, King’s and St Thomas’ Hospitals, London, UK ALBERT FERRO PHD FRCP FBPHARMACOLS Reader in Clinical Pharmacology and Honorary Consultant Physician at King’s College London School of Medicine, Guy’s, King’s and St Thomas’ Hospitals, London, UK PART OF HACHETTE LIVRE UK First published in Great Britain in 1981 Second edition 1986 Third edition 1995 Fourth edition 1999 This fifth edition published in Great Britain in 2008 by Hodder Arnold, an imprint of Hodden Education, part of Hachette Livre UK, 338 Euston Road, London NW1 3BH http://www.hoddereducation.com ©2008 James M Ritter, Lionel D Lewis, Timothy GK Mant and Albert Ferro All rights reserved. Apart from any use permitted under UK copyright law, this publication may only be reproduced, stored or transmitted, in any form, or by any means with prior permission in writing of the publishers or in the case of reprographic production in accordance with the terms of licences issued by the Copyright Licensing Agency. -

Your Pulse and Target Heart Rate
Skip to main content ×Close Menu We use cookies to improve your experience on our website. By closing this banner or interacting with our site, you acknowledge and agree to this. Legal Notices MobileClose Menu Find A Doctor Your Visit Pay a Bill Log in to MyGillette Learn more about MyGillette Patient Education Patient Services and Resources Your Rights and Medical Records Understanding Costs, Insurance and the Gillette Assistance Programs Services to Support Your Family Interpreter Services International Patients Parent Resources and Support Prepare for Your Visit Prepare for Clinic Visits and Tests Prepare for Surgery Take a Hospital Tour Transfer Your Medical Records Transportation and Accommodations Ways to Prepare and Comfort Your Child During Your Visit or Hospital Stay Commitment to a Safe and Healing Environment Our Hospital Units Your Hospital Room St. Paul Campus Amenities and Activities Visit or Contact a Patient Conditions & Care All Conditions Cerebral Palsy Neuromuscular Disorders Scoliosis Craniosynostosis Brain Injury All Tests & Treatments Shunt Surgery for Hydrocephalus Gait and Motion Analysis Selective Dorsal Rhizotomy (SDR) Gillette Craniocap© Orthosis Botulinum Toxin and Phenol (Injected Spasticity Medications) All Specialties & Services Orthopedics Rehabilitation Services Craniofacial and Plastic Surgery Neurology Neurosurgery Virtual Visits Pediatric Expert Consults Virtual Rehab Therapy Sleep Medicine Virtual Care Get Involved Advocating for Your Family Community Health United Cerebral Palsy of MN Donors & Supporters -

Nitric Oxide, the Biological Mediator of the Decade: Fact Or Fiction?
Eur Respir J 1997; 10: 699–707 Copyright ERS Journals Ltd 1997 DOI: 10.1183/09031936.97.10030699 European Respiratory Journal Printed in UK - all rights reserved ISSN 0903 - 1936 SERIES 'CLINICAL PHYSIOLOGY IN RESPIRATORY INTENSIVE CARE' Edited by A. Rossi and C. Roussos Number 14 in this Series Nitric oxide, the biological mediator of the decade: fact or fiction? S. Singh, T.W. Evans Nitric oxide, the biological mediator of the decade: fact or fiction? S. Singh, T.W. Evans. Unit of Critical Care, National Heart & ERS Journals Ltd 1997. Lung Institute, Royal Brompton Hospital, ABSTRACT: Nitric oxide (NO), an atmospheric gas and free radical, is also an London, UK. important biological mediator in animals and humans. Its enzymatic synthesis by constitutive (c) and inducible (i) isoforms of NO synthase (NOS) and its reactions Correspondence: T.W. Evans with other biological molecules such as reactive oxygen species are well charac- Royal Brompton Hospital Sydney Street terized. NO modulates pulmonary and systemic vascular tone through its vasodila- London SW3 6NP tor property. It has antithrombotic functions and mediates some consequences of UK the innate and acute inflammatory responses; cytokines and bacterial toxins induce widespread expression of iNOS associated with microvascular and haemodynam- Keywords: Acute respiratory distress syn- ic changes in sepsis. drome Within the lungs, a diminution of NO production is implicated in pathological hypoxic pulmonary vasoconstriction states associated with pulmonary hypertension, such as acute respiratory distress nitric oxide syndrome: inhaled NO is a selective pulmonary vasodilator and can improve ven- nitric oxide synthase tilation-perfusion mismatch. However, it may have deleterious effects through mod- pulmonary hypertension ulating hypoxic pulmonary vasoconstriction. -

The Heart Institute
The Heart Institute Division of Cardiology 4650 Sunset Blvd., #34, Los Angeles, CA 90027 Phone: 323-361-2461 Fax: 323-361-1513 The Heart Institute at Children’s Hospital Los Angeles is CHLA.org/CARDIOLOGY one of the top-ranked pediatric heart programs in the Division of nation—with a long history of exceptional and innovative Cardiothoracic Surgery 4650 Sunset Blvd., #66 care for the most complex pediatric cardiac conditions. Los Angeles, CA 90027 Phone: 323-361-4148 Fax: 323-361-3668 We treat patients from fetus to adulthood and serve as CHLA.org/CTSurgery a major tertiary referral center for all forms of congenital Referrals Phone: 888-631-2452 and acquired heart disease. Fax: 323-361-8988 Email: [email protected] Physician Portal: https://myCHLA.CHLA.org We offer an integrated inpatient and outpatient Our Cardiothoracic Intensive Care Unit (CTICU) complement of services that brings together experts was the first of its kind on the West Coast, using in cardiology, cardiothoracic surgery, cardiothoracic innovative treatments including extracorporeal transplant, cardiothoracic intensive care and membrane oxygenation (ECMO) and ventricular cardiovascular acute care in a centrally located, assist services. state-of-the-art healing environment. With fewer steps to navigate, our patients and families receive care Our two state-of-the-art catheterization laboratories that is more streamlined and less stressful. use the latest technology to provide accurate cardiac data while reducing radiation. Our programmatic emphasis on high-complexity surgeries in neonates has produced outcomes that are among the best in the country, as shown in the most recent Society of Thoracic Surgeons report. -

Current Advances of Nitric Oxide in Cancer and Anticancer Therapeutics
Review Current Advances of Nitric Oxide in Cancer and Anticancer Therapeutics Joel Mintz 1,†, Anastasia Vedenko 2,†, Omar Rosete 3 , Khushi Shah 4, Gabriella Goldstein 5 , Joshua M. Hare 2,6,7 , Ranjith Ramasamy 3,6,* and Himanshu Arora 2,3,6,* 1 Dr. Kiran C. Patel College of Allopathic Medicine, Nova Southeastern University, Davie, FL 33328, USA; [email protected] 2 John P Hussman Institute for Human Genomics, Miller School of Medicine, University of Miami, Miami, FL 33136, USA; [email protected] (A.V.); [email protected] (J.M.H.) 3 Department of Urology, Miller School of Medicine, University of Miami, Miami, FL 33136, USA; [email protected] 4 College of Arts and Sciences, University of Miami, Miami, FL 33146, USA; [email protected] 5 College of Health Professions and Sciences, University of Central Florida, Orlando, FL 32816, USA; [email protected] 6 The Interdisciplinary Stem Cell Institute, Miller School of Medicine, University of Miami, Miami, FL 33136, USA 7 Department of Medicine, Cardiology Division, Miller School of Medicine, University of Miami, Miami, FL 33136, USA * Correspondence: [email protected] (R.R.); [email protected] (H.A.) † These authors contributed equally to this work. Abstract: Nitric oxide (NO) is a short-lived, ubiquitous signaling molecule that affects numerous critical functions in the body. There are markedly conflicting findings in the literature regarding the bimodal effects of NO in carcinogenesis and tumor progression, which has important consequences for treatment. Several preclinical and clinical studies have suggested that both pro- and antitumori- Citation: Mintz, J.; Vedenko, A.; genic effects of NO depend on multiple aspects, including, but not limited to, tissue of generation, the Rosete, O.; Shah, K.; Goldstein, G.; level of production, the oxidative/reductive (redox) environment in which this radical is generated, Hare, J.M; Ramasamy, R.; Arora, H. -

Medical Management of Advanced Heart Failure
CLINICAL CARDIOLOGY CLINICIAN’S CORNER Medical Management of Advanced Heart Failure Anju Nohria, MD Context Advanced heart failure, defined as persistence of limiting symptoms de- Eldrin Lewis, MD spite therapy with agents of proven efficacy, accounts for the majority of morbidity and mortality in heart failure. Lynne Warner Stevenson, MD Objective To review current medical therapy for advanced heart failure. EART FAILURE HAS EMERGED AS Data Sources We searched MEDLINE for all articles containing the term advanced a major health challenge, in- heart failure that were published between 1980 and 2001; EMBASE was searched from creasing in prevalence as age- 1987-1999, Best Evidence from 1991-1998, and Evidence-Based Medicine from 1995- adjusted rates of myocardial 1999. The Cochrane Library also was searched for critical reviews and meta-analyses Hinfarction and stroke decline.1 Affect- of congestive heart failure. ing 4 to 5 million people in the United Study Selection Randomized controlled trials of therapy for 150 patients or more States with more than 2 million hospi- were included if advanced heart failure was represented. Other common clinical situ- talizations each year, heart failure alone ations were addressed from smaller trials as available, trials of milder heart failure, con- accounts for 2% to 3% of the national sensus guidelines, and both published and personal clinical experience. health care budget. Developments her- Data Extraction Data quality was determined by publication in peer-reviewed lit- alded in the news media increase pub- erature or inclusion in professional society guidelines. lic expectations but focus on decreas- Data Synthesis A primary focus for care of advanced heart failure is ongoing iden- ing disease progression in mild to tification and treatment of the elevated filling pressures that cause disabling symp- moderate stages2,3 or supporting the cir- toms. -

The Evolution of Heart Failure with Reduced Ejection Fraction Pharmacotherapy: What Do We Have and Where Are We Going?
Pharmacology & Therapeutics 178 (2017) 67–82 Contents lists available at ScienceDirect Pharmacology & Therapeutics journal homepage: www.elsevier.com/locate/pharmthera Associate editor: M. Curtis The evolution of heart failure with reduced ejection fraction pharmacotherapy: What do we have and where are we going? Ahmed Selim, Ronald Zolty, Yiannis S. Chatzizisis ⁎ Division of Cardiovascular Medicine, University of Nebraska Medical Center, Omaha, NE, USA article info abstract Available online 21 March 2017 Cardiovascular diseases represent a leading cause of mortality and increased healthcare expenditure worldwide. Heart failure, which simply describes an inability of the heart to meet the body's needs, is the end point for many Keywords: other cardiovascular conditions. The last three decades have witnessed significant efforts aiming at the discovery Heart failure of treatments to improve the survival and quality of life of patients with heart failure; many were successful, Reduced ejection fraction while others failed. Given that most of the successes in treating heart failure were achieved in patients with re- Pharmacotherapy duced left ventricular ejection fraction (HFrEF), we constructed this review to look at the recent evolution of Novel drugs HFrEF pharmacotherapy. We also explore some of the ongoing clinical trials for new drugs, and investigate poten- tial treatment targets and pathways that might play a role in treating HFrEF in the future. © 2017 Elsevier Inc. All rights reserved. Contents 1. Introduction.............................................. -
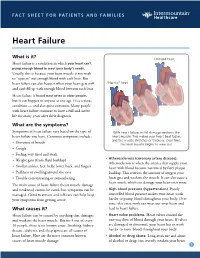
Heart Failure
FACT SHEET FOR PATIENTS AND FAMILIES Heart Failure What is it? Enlarged heart Heart failure is a condition in which your heart can’t pump enough blood to meet your body’s needs. Usually, this is because your heart muscle is too weak to “squeeze” out enough blood with each beat. But heart failure can also happen when your heart gets stiff “Normal” heart and can’t fill up with enough blood between each beat. Heart failure is found most often in older people, but it can happen to anyone at any age. It’s a serious condition — and also quite common. Many people with heart failure continue to have a full and active life for many years after their diagnosis. What are the symptoms? Symptoms of heart failure vary based on the type of With heart failure, initial damage weakens the heart failure you have. Common symptoms include: heart muscle. This makes your heart beat faster, and the muscle stretches or thickens. Over time, • Shortness of breath the heart muscle begins to wear out. • Cough • Feeling very tired and weak • Atherosclerosis (coronary artery disease). • Weight gain (from fluid buildup) Atherosclerosis is when the arteries that supply your • Swollen ankles, feet, belly, lower back, and fingers heart with blood become narrowed by fatty plaque • Puffiness or swelling around the eyes buildup. This restricts the amount of oxygen your • Trouble concentrating or remembering heart gets and weakens the muscle. It can also cause a heart attack, which can damage your heart even more. The main cause of heart failure (heart muscle damage and weakness) cannot be cured, but symptoms can be • High blood pressure (hypertension). -

Investigations Into the Role of Nitric Oxide in Disease
INVESTIGATIONS INTO THE ROLE OF NITRIC OXIDE IN CARDIOVASCULAR DEVXLOPMENT DISEASE: INSIGHTS GAINED FROM GENETICALLY ENGINEERED MOUSE MODELS OF HUMAN DISEASE Tony Lee A thesis submitted in conformity with the requirements for the degree of Master's of Science Institute of Medicai Science University of Toronto @ Copyright by Tony C. Lee (2000) National Library Bibliothèque nationale I*I of Canada du Canada Acquisitions and Acquisitions et Bibliogaphic Services services bibliogaphiques 395 Wellington Street 395, rue Wellington Ottawa ON K1A ON4 Ottawa ON KIA ON4 Canada Canada The author has granted a non- L'auteur a accordé une licence non exclusive licence allowing the exclusive permettant à la National Library of Canada to Bibliothèque nationale du Canada de reproduce, loan, distribute or sell reproduire, prêter, distribuer ou copies of this thesis in microfonn, vendre des copies de cette thèse sous paper or electronic formats. la forme de microfiche/nlm, de reproduction sur papier ou sur format électronique. The author retains ownership of the L'auteur conserve la propriété du copyright in this thesis. Neither the droit d'auteur qui protège cette thèse. thesis nor substantial extracts fkom it Ni la thèse ni des extraits substantiels may be printed or othewise de celle-ci ne doivent être imprimés reproduced without the author's ou autrement reproduits sans son permission. autorisation. INVESTIGATIONS INTO THE ROLE OF MTNC OXIDE IN CARDIOVASCULAR DEVELOPMENT AND DISEASE: INSIGHTS GAiNED FROM GENETICALLY ENGINEERED MOUSE MODELS OF HUMAN DISEASE B y Tony Lee A thesis submitted in conformity with the requirements for the degree of Master's of Science (2000) Instinite of Medical Science, University of Toronto In the first expenmental series, the antiatherosclerotic effects of Enalûpril vrrsus Irbesartan were compared in the LDL-R knockout mouse model. -

Hair Growth Stimulation with Nitroxide and Other Radicals
Europaisches Patentamt 263 European Patent Office © Publication number: 0 327 Office europeen des brevets A1 © EUROPEAN PATENT APPLICATION © Application number: 89300785.6 © Int. CI.4: A61 K 7/06 , A61K 47/00 © Date of filing: 27.01.89 © Priority: 29.01.88 US 149720 © Applicant: PROCTOR, Peter H. Twelve Oaks Medical Tower 4125 Southwest © Date of publication of application: Freeway 09.08.89 Bulletin 89/32 Suite 1616 Houston, TX 77027(US) © Designated Contracting States: © Inventor: PROCTOR, Peter H. AT BE CH OE ES FR GB GR IT Li LU NL SE Twelve Oaks Medical Tower 4125 Southwest Freeway Suite 1616 Houston, TX 77027(US) © Representative: Gore, Peter Manson et al W.P. THOMPSON & CO. Coopers Building Church Street Liverpool L1 3AB(GB) © Hair growth stimulation with nitroxide and other radicals. © Hair growth stimulation with nitroxide and other radicals. A nitroxide radical source compound is applied topically with an adjuvant selected from reducing agents, hydroxyl radical scavengers and antioxidants to activate formation of the nitroxide radical and/or to protect the nitroxide radical from reaction with other free radicals. Also disclosed is a kit for preparing a unit dose for topical application of a nitric oxide generating compound and a reducing agent reactive therewith to form nitric oxide on or in the skin. Other adjuvants include SOD and antiandrogens. Various other radical-forming hair growth stimulants and preparations thereof are disclosed. < w CO CM IN CM CO © Q. Ill Xerox Copy Centre EP 0 327 263 A1 rIAIR GROWTH STIMULATION WITH NITROXIDE AND OTHER RADICALS This invention relates to a composition and method for stimulating hair growth, and to a kit for preparation of such a composition. -

Grade 6: the Heart and Circulatory System Lesson 1: the Heart Lesson 2: the Heart Rate Lesson 3: the Circulatory System and Blood
Grade 6: The Heart and Circulatory System Lesson 1: The Heart Lesson 2: The Heart Rate Lesson 3: The Circulatory System and Blood Objectives: 1. Students will identify the four chambers of the heart 2. Students will identify four important structures of the Circulatory System and what they do. 3. Students will explain heart rate and be able to take their resting and active heart rates. 4. Students will describe the major functions of the Circulatory System. 5. Students will explain the role of the heart in circulation 6. Students will give a basic explanation of the cardio-pulmonary sequence. 7. Students will describe systemic circulation. Materials: Lesson 1: • Animal heart (Example: cow, pig, sheep) • Note cards • Picture of the heart (See Figure 1) • Dissection tools (Scissors, pan, etc.) Lesson 2: • Small drum • Watch or clock with second hand, or time • Optional: Stethoscope Lesson 3: • Corn Syrup • Plastic Beads: flat red disks, white ovals, green or blue seed beads • “Explain” experiment (per group): o Two small balloons or large finger cots o One clear tube (1/2” diameter) about 8” long o One clear tube (3/4” diameter) about 8” long o 16 - 20 oz. water o Red food coloring o Measuring cup o Funnel o Two empty plastic containers (such as cottage cheese or yogurt cartons) Activity Summary: In this lesson students will learn the basic functioning of the heart, Circulatory System and blood, the connection to lung functioning, and the activity of the Grade 6: The Heart & Circulatory System – Revised 2008 Page 1 Circulatory System in the body.