Identification of Polymorphism in Ethylone Hydrochloride: Synthesis and Characterization
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Application of High Resolution Mass Spectrometry for the Screening and Confirmation of Novel Psychoactive Substances Joshua Zolton Seither [email protected]
Florida International University FIU Digital Commons FIU Electronic Theses and Dissertations University Graduate School 4-25-2018 Application of High Resolution Mass Spectrometry for the Screening and Confirmation of Novel Psychoactive Substances Joshua Zolton Seither [email protected] DOI: 10.25148/etd.FIDC006565 Follow this and additional works at: https://digitalcommons.fiu.edu/etd Part of the Chemistry Commons Recommended Citation Seither, Joshua Zolton, "Application of High Resolution Mass Spectrometry for the Screening and Confirmation of Novel Psychoactive Substances" (2018). FIU Electronic Theses and Dissertations. 3823. https://digitalcommons.fiu.edu/etd/3823 This work is brought to you for free and open access by the University Graduate School at FIU Digital Commons. It has been accepted for inclusion in FIU Electronic Theses and Dissertations by an authorized administrator of FIU Digital Commons. For more information, please contact [email protected]. FLORIDA INTERNATIONAL UNIVERSITY Miami, Florida APPLICATION OF HIGH RESOLUTION MASS SPECTROMETRY FOR THE SCREENING AND CONFIRMATION OF NOVEL PSYCHOACTIVE SUBSTANCES A dissertation submitted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY in CHEMISTRY by Joshua Zolton Seither 2018 To: Dean Michael R. Heithaus College of Arts, Sciences and Education This dissertation, written by Joshua Zolton Seither, and entitled Application of High- Resolution Mass Spectrometry for the Screening and Confirmation of Novel Psychoactive Substances, having been approved in respect to style and intellectual content, is referred to you for judgment. We have read this dissertation and recommend that it be approved. _______________________________________ Piero Gardinali _______________________________________ Bruce McCord _______________________________________ DeEtta Mills _______________________________________ Stanislaw Wnuk _______________________________________ Anthony DeCaprio, Major Professor Date of Defense: April 25, 2018 The dissertation of Joshua Zolton Seither is approved. -

Model Scheduling New/Novel Psychoactive Substances Act (Third Edition)
Model Scheduling New/Novel Psychoactive Substances Act (Third Edition) July 1, 2019. This project was supported by Grant No. G1799ONDCP03A, awarded by the Office of National Drug Control Policy. Points of view or opinions in this document are those of the author and do not necessarily represent the official position or policies of the Office of National Drug Control Policy or the United States Government. © 2019 NATIONAL ALLIANCE FOR MODEL STATE DRUG LAWS. This document may be reproduced for non-commercial purposes with full attribution to the National Alliance for Model State Drug Laws. Please contact NAMSDL at [email protected] or (703) 229-4954 with any questions about the Model Language. This document is intended for educational purposes only and does not constitute legal advice or opinion. Headquarters Office: NATIONAL ALLIANCE FOR MODEL STATE DRUG 1 LAWS, 1335 North Front Street, First Floor, Harrisburg, PA, 17102-2629. Model Scheduling New/Novel Psychoactive Substances Act (Third Edition)1 Table of Contents 3 Policy Statement and Background 5 Highlights 6 Section I – Short Title 6 Section II – Purpose 6 Section III – Synthetic Cannabinoids 13 Section IV – Substituted Cathinones 19 Section V – Substituted Phenethylamines 23 Section VI – N-benzyl Phenethylamine Compounds 25 Section VII – Substituted Tryptamines 28 Section VIII – Substituted Phenylcyclohexylamines 30 Section IX – Fentanyl Derivatives 39 Section X – Unclassified NPS 43 Appendix 1 Second edition published in September 2018; first edition published in 2014. Content in red bold first added in third edition. © 2019 NATIONAL ALLIANCE FOR MODEL STATE DRUG LAWS. This document may be reproduced for non-commercial purposes with full attribution to the National Alliance for Model State Drug Laws. -

Agenda Florida Hospital Association 307 Park Lake Circle Orlando, FL July 14, 2016 @ 2:00 P.M
The Florida Board of Nursing Controlled Substances Formulary Committee Agenda Florida Hospital Association 307 Park Lake Circle Orlando, FL July 14, 2016 @ 2:00 p.m. Doreen Cassarino, DNP, ARNP, FNP-BC, BC- ADM, FAANP - Chair Joe Baker, Jr. Executive Director Kathryn L Controlled Substances Formulary Committee Agenda July 14, 2016 @ 2:00 p.m. Committee Members: Doreen Cassarino, DNP, FNP-BC, BC-ADM, FAANP (Chair) Vicky Stone-Gale, DNP, FNP-C, MSN Jim Quinlan, DNP, ARNP Bernardo B. Fernandez, Jr., MD, MBA, FACP Joshua D. Lenchus, DO, RPh, FACP, SFHM Eduardo C. Oliveira, MD, MBA, FCCP Jeffrey Mesaros, PharmD, JD Attorney: Lee Ann Gustafson, Senior Assistant Attorney General Board Staff: Joe Baker, Jr., Executive Director Jessica Hollingsworth, Program Operations Administrator For more information regarding board meetings please visit http://floridasnursing.gov/meeting-information/ Or contact: Florida Board of Nursing 4052 Bald Cypress Way, Bin # C-02 Tallahassee, FL 32399-3252 Direct Line: (850)245-4125/Direct Fax: (850)617-6450 Email: [email protected] Call to Order Roll Call Committee Members: Doreen Cassarino, DNP, FNP-BC, BC-ADM, FAANP (Chair) Vicky Stone-Gale, DNP, FNP-C, MSN Jim Quinlan, DNP, ARNP Bernardo B. Fernandez, Jr., MD, MBA, FACP Joshua D. Lenchus, DO, RPh, FACP, SFHM Eduardo C. Oliveira, MD, MBA, FCCP Jeffrey Mesaros, PharmD, JD Attorney: Lee Ann Gustafson, Senior Assistant Attorney General Board Staff: Joe Baker, Jr., Executive Director Jessica Hollingsworth, Program Operations Administrator I. Review & Approve Minutes from June 29, 2016 Meeting II. Open Discussion A. Recommendations to the Board of Nursing for Rule Promulgation B. Reference Material 1. -

Long-Term Stability of Synthetic Cathinones in Forensic Toxicology Samples Author(S): Sarah Kerrigan, Ph.D., Lindsay Glicksberg, B.S
NCJRS OFFICE OF JUSTICE PROGRAMS ~ N ATIONAL CRIMINAL JUSTICE REFERENCE SERVICE QJA BJS N/J OJJ[l> OVC SMART ",iiiii~ The author(s) shown below used Federal funding provided by the U.S. Department of Justice to prepare the following resource: Document Title: Long-Term Stability of Synthetic Cathinones in Forensic Toxicology Samples Author(s): Sarah Kerrigan, Ph.D., Lindsay Glicksberg, B.S. Document Number: 251194 Date Received: October 2017 Award Number: 2013-R2-CX-K006 This resource has not been published by the U.S. Department of Justice. This resource is being made publically available through the Office of Justice Programs’ National Criminal Justice Reference Service. Opinions or points of view expressed are those of the author(s) and do not necessarily reflect the official position or policies of the U.S. Department of Justice. Contents Abstract........................................................................................................................................... 4 Executive Summary ........................................................................................................................ 6 I. Introduction .......................................................................................................................... 10 Statement of the Problem ........................................................................................................ 10 Literature Citations and Review ................................................................................................ 11 Use and -

Drugs and New Potentially Dangerous Chemical Substances, with a Brief Review of the Problem
INTERNATIONAL JOURNAL OF ENVIRONMENTAL & SCIENCE EDUCATION 2016, VOL. 11, NO. 14, 6697-6703 OPEN ACCESS Approach to Classifying “Design” Drugs and New Potentially Dangerous Chemical Substances, With a Brief Review of the Problem Azat R. Asadullina, Elena Kh. Galeevab, Elvina A. Achmetovaa and Ivan V. Nikolaevb aBashkir State Medical University of the Ministry of Health of the Russian Federation, Ufa, RUSSIA; bRepublican Narcological Dispensary No.1 of the Ministry of Health of the Republic of Bashkortostan, Ufa, RUSSIA ABSTRACT The urgency of this study has become vivid in the light of the growing problem of prevalence and bBPHFuse Republican of new synthetic Narcological drug types. DispensaryLately there has No.1 been of a thetendency Ministry of expanding of Health the rangeof the of psychologically active substances (PAS) used by addicts with the purpose of their illegal taking. RepublicThe aim of Bashkortostan,of this research is anPushkin attempt str.,of systematizing 119/1, Ufa, and RB. classifying “design” drugs according to their chemical structure, neurochemical mechanisms of action and clinical manifestations. As a result, we have found that they can be divided into ten big groups. This classification will allow to better arrange new clinical phenomenology in modern addictology. This paper would be useful for psychiatrists, experts in narcology, as well as for personnel of institutions and agencies engaged in anti-drug activity. KEYWORDS ARTICLE HISTORY Opiates, cannabinoids, cathinones, tryptamine, Received 20 April 2016 cocaine, pregabalin, new “design” drugs, Revised 28 May 2016 classification Accepted 29 Мау 2016 Introduction Urgency of the problem Recently, the sphere of illegal turnover of narcotic substances has shown an apparent trend of producing the so-called “design drugs” (DN): new potentially dangerous psychologically active substances (NPDPAS) obtained by means of chemical synthesis, possessing a high narcotic effect and manufactured with the CORRESPONDENCE Azat R. -

Molly Myth Exposed Over the Past Three-Years, Drug Traffickers And
Molly Myth Exposed Over the past three-years, drug traffickers and music festivals have been promoting the illegal street drug “Molly” as being “pure MDMA.” While some drugs sold as Molly might be MDMA or contain some MDMA in combination with other drugs, at least in South Florida 1,194 samples of drugs called Mollys in 2013 had no MDMA but contained the now illegal, former “bath salt,” methylone (or bk-3,4 methylenedioxy-methcathinone). Methylone belongs to the group of illicit stimulants known as synthetic cathinones. Between 2011 and 2013 the number of crime lab reports in South Florida (Miami-Dade, Broward, and Palm Beach Counties) for actual MDMA decreased 82-percent from 299 to 54 while reports for synthetic cathinones increased 2,857-percent from 42 to 1,242 including the aforementioned 1,194 specifically for methylone. In just the first six months of 2014, there were 72 deaths across Florida related to synthetic cathinones with 19 of them considered to be caused by the drug. Beginning in 2014, methylone is being replaced with another synthetic cathinone, ethylone, as the drug sold in Mollys. The National crime lab reports for 2013 released by the US-Drug Enforcement Administration reveals a 54-percent decline in actual MDMA reports from 10,332 in 2011 to 4,798 in 2013 and a 137-percent increase in synthetic cathinones from 6,949 to 16,500. Methylone and ethylone may be purchased illegally from China on the Internet and chipped into small crystal pieces placed in capsules. Dosage levels may vary dramatically from one pill to another. -

Uniform Controlled Substances Act
CHAPTER 19-03.1 UNIFORM CONTROLLED SUBSTANCES ACT 19-03.1-01. Definitions. As used in this chapter and in chapters 19-03.2 and 19-03.4, unless the context otherwise requires: 1. "Administer" means to apply a controlled substance, whether by injection, inhalation, ingestion, or any other means, directly to the body of a patient or research subject by: a. A practitioner or, in the practitioner's presence, by the practitioner's authorized agent; or b. The patient or research subject at the direction and in the presence of the practitioner. 2. "Agent" means an authorized person who acts on behalf of or at the direction of a manufacturer, distributor, or dispenser. It does not include a common or contract carrier, public warehouseman, or employee of the carrier or warehouseman. 3. "Anabolic steroids" means any drug or hormonal substance, chemically and pharmacologically related to testosterone, other than estrogens, progestins, and corticosteroids. 4. "Board" means the state board of pharmacy. 5. "Bureau" means the drug enforcement administration in the United States department of justice or its successor agency. 6. "Controlled substance" means a drug, substance, or immediate precursor in schedules I through V as set out in this chapter. 7. "Controlled substance analog": a. Means a substance the chemical structure of which is substantially similar to the chemical structure of a controlled substance in a schedule I or II and: (1) Which has a stimulant, depressant, or hallucinogenic effect on the central nervous system which is substantially similar to or greater than the stimulant, depressant, or hallucinogenic effect on the central nervous system of a controlled substance in schedule I or II; or (2) With respect to a particular individual, which the individual represents or intends to have a stimulant, depressant, or hallucinogenic effect on the central nervous system substantially similar to or greater than the stimulant, depressant, or hallucinogenic effect on the central nervous system of a controlled substance in schedule I or II. -

In the United States District Court for the District Of
Case 1:15-cr-00245-SOM Document 82 Filed 04/22/16 Page 1 of 23 PageID #: <pageID> IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF HAWAII UNITED STATES OF AMERICA, ) CR. NO. 15-00245-01 SOM ) CR. NO. 15-00410-01 SOM Plaintiff, ) ) ORDER DETERMINING THAT, FOR vs. ) PURPOSES OF CALCULATING THE ) SENTENCING GUIDELINE RANGE, AUSTIN-ERNEST KAHOLO HOLMES, ) METHCATHINONE IS THE LISTED ) DRUG MOST CLOSELY RELATED TO Defendant. ) ETHYLONE _____________________________ ) ORDER DETERMINING THAT, FOR PURPOSES OF CALCULATING THE SENTENCING GUIDELINE RANGE, METHCATHINONE IS THE LISTED DRUG MOST CLOSELY RELATED TO ETHYLONE This court is asked to determine which drug listed in the Drug Equivalency Tables of § 2D1.1 of the United States Sentencing Guidelines is most closely related to ethylone, a drug not mentioned in the Sentencing Guidelines. Having heard from dueling scientists during two days of testimony, this court agrees with Defendant Austin-Ernest Kaholo Homes that methcathinone is the listed drug most closely related to ethylone. This conclusion relates solely to the calculation of the guideline range applicable in this case, not to whether, once the guideline range has been determined, there may or may not be good reasons for this court to vary from that guideline range, a matter the court will address at an upcoming hearing. That is, this court is not here addressing any challenge to how the Sentencing Guidelines characterize methcathinone or any argument Case 1:15-cr-00245-SOM Document 82 Filed 04/22/16 Page 2 of 23 PageID #: <pageID> as to how this court should ultimately treat ethylone, although the court fully expects such challenges and arguments to be raised in connection with a defense request for a variance from the guideline range.1 I. -

Template 1..106
CHAPTER 2018-13 Committee Substitute for Committee Substitute for House Bill No. 21 An act relating to controlled substances; creating s. 456.0301, F.S.; requiring certain boards to require certain registered practitioners to complete a specified board-approved continuing education course to obtain author- ization to prescribe controlled substances as part of biennial license renewal and before a specified date; providing course requirements; providing that the course may be offered in a distance learning format and requiring that it be included within required continuing education hours; prohibiting the Department of Health from renewing the license of a prescriber under specified circumstances; specifying a deadline for course completion; providing an exception from the course requirements for certain licensees; requiring such licensees to submit confirmation of course completion; authorizing certain boards to adopt rules; amending s. 456.072, F.S.; authorizing disciplinary action against practitioners for violating specified provisions relating to controlled substances; amending s. 456.44, F.S.; defining the term “acute pain”; requiring the applicable boards to adopt rules establishing certain guidelines for prescribing controlled substances for acute pain; providing that the failure of a prescriber to follow specified guidelines is grounds for disciplinary action; limiting opioid drug prescriptions for the treatment of acute pain to a specified period under certain circumstances; authorizing such prescrip- tions for an extended period if specified requirements are met; requiring a prescriber who prescribes an opioid drug for the treatment of pain other than acute pain to include a specific indication on the prescription; requiring a prescriber who prescribes an opioid drug for the treatment of pain related to a traumatic injury with a specified Injury Severity Score to concurrently prescribe an emergency opioid antagonist; amending ss. -
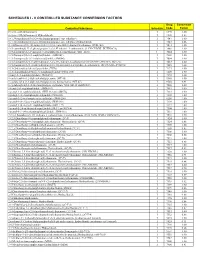
Controlled Substance Conversion Factors
SCHEDULES I - V CONTROLLED SUBSTANCE CONVERSION FACTORS Drug Conversion Controlled Substance Schedule Code Factor (+/-)cis -4-Methylaminorex I 1590 1.00 (+/-)cis -4-Methylaminorex Hydrochloride I 1590 0.83 1-(1,3-benzodioxol-5-yl)-2-(ethylamino)propan-1-one (ethylone) I 7547 1.00 1-(1,3-benzodioxol-5-yl)-2-(ethylamino)propan-1-one (ethylone) hydrochloride I 7547 0.86 (1-(4-fluorobenzyl)-1H -indol-3-yl)(2,2,3,3-tetramethylcyclopropyl)methanone (FUB-144) I 7014 1.00 1-(4-cyanobutyl)-N -(2-phenylpropan-2-yl)-1H -indazole-3-carboxamide (4-CN-CUMYL-BUTINACA) I 7089 1.00 1-(5-fluoropentyl)-1H -indazol-3-yl](naphthalen-1-yl)methanone (THJ–2201) 1 7024 1.00 1-(5-fluoropentyl)-3-(1-naphthoyl)indole (AM2201) I 7201 1.00 1-(5-fluoropentyl)-3-(2-iodobenzoyl)indole (AM694) I 7694 1.00 1-(5-fluoropentyl)-N -(2-phenylpropan-2-yl)-1H -indazole-3-carboxamide (5F-CUMYL-PINACA; SGT-25) I 7083 1.00 1-(5-fluoropentyl)-N -(2-phenylpropan-2-yl)-1H -pyrrolo[2,3-b]pyridine-3-carboxamide (5F-CUMYL-P7AICA) I 7085 1.00 1-[1-(2-thienyl)cyclohexyl]pyrrolidine (TCPy) I 7473 1.00 1-[2-(4-morpholinyl)ethyl]-3-(1-naphthoyl)indole (JWH- 200) I 7200 1.00 1-butyl-3-(1-naphthoyl)indole (JWH-073) I 7173 1.00 1-cyclohexyl-4-(1,2-diphenylethyl)piperazine (MT-45) I 9560 1.00 1-cyclohexyl-4-(1,2-diphenylethyl)piperazine hydrochloride (MT-45) I 9560 0.91 1-cyclohexylethyl-3-(2-methoxyphenylacetyl)indole 7008 (SR-18 and RCS-8) I 7008 1.00 1-hexyl-3-(1-naphthoyl)indole (JWH-019) I 7019 1.00 1-pentyl-3-(1-naphthoyl)indole (JWH-018 and AM678) I 7118 1.00 1-pentyl-3-(2-chlorophenylacetyl)indole -

House Bill 2453
House Engrossed State of Arizona House of Representatives Fifty-first Legislature Second Regular Session 2014 HOUSE BILL 2453 AN ACT AMENDING SECTIONS 13-3401 AND 13-3404, ARIZONA REVISED STATUTES; RELATING TO DRUG OFFENSES. (TEXT OF BILL BEGINS ON NEXT PAGE) - i - H.B. 2453 1 Be it enacted by the Legislature of the State of Arizona: 2 Section 1. Section 13-3401, Arizona Revised Statutes, is amended to 3 read: 4 13-3401. Definitions 5 In this chapter, unless the context otherwise requires: 6 1. "Administer" means to apply, inject or facilitate the inhalation or 7 ingestion of a substance to the body of a person. 8 2. "Amidone" means any substance identified chemically as 9 (4-4-diphenyl-6-dimethylamine-heptanone-3), or any salt of such substance, by 10 whatever trade name designated. 11 3. "Board" means the Arizona state board of pharmacy. 12 4. "Cannabis" means the following substances under whatever names they 13 may be designated: 14 (a) The resin extracted from any part of a plant of the genus 15 cannabis, and every compound, manufacture, salt, derivative, mixture or 16 preparation of such plant, its seeds or its resin. Cannabis does not include 17 oil or cake made from the seeds of such plant, any fiber, compound, 18 manufacture, salt, derivative, mixture or preparation of the mature stalks of 19 such plant except the resin extracted from the stalks or any fiber, oil or 20 cake or the sterilized seed of such plant which is incapable of germination. 21 (b) Every compound, manufacture, salt, derivative, mixture or 22 preparation of such resin or tetrahydrocannabinol. -

Day 1 Printable Slides
Herbal Smoking Blends - SPICE • SPICE and other herbal blends have been sold in head shops and on the Internet since 2006 for their cannabis-like intoxication Classes and Structures of Emerging Cannabimimetics • The herbal blends sometimes have a fragrance and Cathinones which could include vanilla, potpourri, spice, blueberry, caramel, and strawberry Arthur Berrier Senior Research Chemist Special Testing and Research Laboratory • The plant materials for the different blends Drug Enforcement Administration have a wide variation in appearance ~ ) D[A Speco0I Tornnr. oncl lle1ea1ch 1.1boratory ~~ Emt!•t;mg Trends Ptobr,1m Herbal Smoking Blends - SPICE Herbal Smoking Blends - SPICE • In late 2008, THC Pharma reported the • K2 enters market in April, 2009 presence of JWH 018, a synthetic cannabimimetic indole in some blends • Interest booms in herbal smoking blends in 2009 • In early 2009, analogues of CP 47,497 (another synthetic cannabinoid) were also • Many new products appeared, typically with found in some blends by the U. of Freiburg JWH 018 and JWH 073 • In early 2009, several European Countries • States begin to control the blends/synthetic control Herbal Blends/JWH 018/CP 47,497 cannabinoids ~) 0(1\ Spr•c:i.11 f~l'.. [1 ng and Rtl~C!'allh l11hor,1to ry ~ ' ) O(I' S1Jec1a l Tl.?sltng amJ RPWJr< h l .1bor,1tory r"::::> Fme rf;mg Trt>nd. Proc;f.101 ~=> [me1eing Tr!>nth P1ogr.i111 Classes of Synthetic Cannabinoids Herbal Smoking Blends - SPICE Observed on Smoking Blends • Products quickly reformulated with new, non i) 2-(3-hydroxycyclohexyl)phenol (CP 47,497) controlled synthetic cannabimimetics ii) 3-(1-naphthoyl)indole (JWH 018) iii) 3-(1-naphthoyl)pyrrole • Five of the synthetic cannabimimetics iv) 1-( 1-naphthylmethylene )indene controlled at the Federal level in 2011 v) 3-phenylacetylindole or 3-benzoylindole • Synthetic Drug Abuse Prevention Act of 2012 ~ ) 11[ •\ "' 1,r( 1,-,1 lf'.',..tr11g and [~p Pnrch lJUOrdto1 y ~ \ DE/\ Special Te1 1ing .1nrJ R·~>P<Hrh I abora\Clly r C :> f 1n~~ 1 ,_,1 r\ , Trc11ds P1L)grJm -r.:---::::>J fniPre1ng lrend'.