Overview of the Biological Characteristics of Alewife (Alosa Pseudoharengus)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Barriers to Fish Passage in Nova Scotia the Evolution of Water Control Barriers in Nova Scotia’S Watershed
Dalhousie University- Environmental Science Barriers to Fish Passage in Nova Scotia The Evolution of Water Control Barriers in Nova Scotia’s Watershed By: Gillian Fielding Supervisor: Shannon Sterling Submitted for ENVS 4901- Environmental Science Honours Abstract Loss of connectivity throughout river systems is one of the most serious effects dams impose on migrating fish species. I examine the extent and dates of aquatic habitat loss due to dam construction in two key salmon regions in Nova Scotia: Inner Bay of Fundy (IBoF) and the Southern Uplands (SU). This work is possible due to the recent progress in the water control structure inventory for the province of Nova Scotia (NSWCD) by Nova Scotia Environment. Findings indicate that 586 dams have been documented in the NSWCD inventory for the entire province. The most common main purpose of dams built throughout Nova Scotia is for hydropower production (21%) and only 14% of dams in the database contain associated fish passage technology. Findings indicate that the SU is impacted by 279 dams, resulting in an upstream habitat loss of 3,008 km of stream length, equivalent to 9.28% of the total stream length within the SU. The most extensive amount of loss occurred from 1920-1930. The IBoF was found to have 131 dams resulting in an upstream habitat loss of 1, 299 km of stream length, equivalent to 7.1% of total stream length. The most extensive amount of upstream habitat loss occurred from 1930-1940. I also examined if given what I have learned about the locations and dates of dam installations, are existent fish population data sufficient to assess the impacts of dams on the IBoF and SU Atlantic salmon populations in Nova Scotia? Results indicate that dams have caused a widespread upstream loss of freshwater habitat in Nova Scotia howeverfish population data do not exist to examine the direct impact of dam construction on the IBoF and SU Atlantic salmon populations in Nova Scotia. -

Tusket River & Basin
Tusket River & Basin Introduction The Tusket River presents an area of 3000 square kilometres (1158 square miles) on the southwestern tip of Nova Scotia. The area consists of a coastal basin with an inland watershed associated with the great Tusket River. This name is derived from a Mi'kmaq word "Neketaouksit" meaning "Great Forked Tidal River". This unique and diverse environment has offered natural resources to the Mi'kmaq First Nation's people for 7000 years and to the French Acadian settlers since the early 17th century. In the context of sustainable development, the present-day Acadians can offer a bilingual ecotourism package of nature-adventure and discovery blended with local history and heritage. The following natural history of the area will be presented in two segments. The first section will describe the natural environment of the Tusket Basin and its interrelationships with the Acadian people. The second segment will describe the natural history of the Tusket River in a similar manner to present the Acadian in his natural environment. Basin description 1. Physical features The Tusket Basin has a width of 32 kilometres (20 miles) between headlands at Chebogue Point and Lower East Pubnico. The mainland coastline between these two boundaries is highly indented and irregular with a measure of 500 kilometres (310 miles). Elongated points, peninsulas, ridges, drumlins (low hills) and eskers are oriented North-South and are separated by many tidal channels, inlets, estuaries and bays. High tide in the estuary of the "great forked tidal river" (Tusket) carries salt water inland for 24 kilometres (15 miles). -

Labour Day 2011 Returns to the Gaspereau Valley Wolfville, Nova Scotia September 2 to 5, 2011
Labour Day 2011 Returns to the Gaspereau Valley Wolfville, Nova Scotia September 2 to 5, 2011 Long time members of the Maritime Organization of Rover Enthusiasts (MORE) will remember Labour Day in and around Wolfville back in 1999 and 2000. After years of resistance we, Julie and Peter Rosvall, will host Labour Day on our property once again. With the help of fellow Rover owners in our neighbourhood we will invite folks from across the Maritimes, New England, points west and internationally to our home. Kris Lockhart has been scouting and doing trail maintenance for so long already that the local joke is that by Labour Day 2011 he might have them paved if we don't watch out. All trailheads will be within 5 minutes of our property, and will have lots of options for every driving preference. Rosie Browning is taking the lead on food, after the great success of her desserts in Cape Breton. She has dreams of sandwiches made with roast beef and turkey (yes, real roast, really, cooked just prior to making the sandwiches, I'm drooling already). Rosie will be helped by a team of my relatives and our neighbours. All meals from Friday evening to Sunday night are provided on site, and the food will be amazing. I'm sure we'll have the Barr family put to work on this and other logistical details, and the Rudermans as well, and anyone else we can sucker in to volunteering leading up to the event and during. (willing volunteers can contact me using the coordinates below) Camping for the event will be on our property; tents will be setup among the cherry and pear trees, bordered by blackberry bushes. -

Minas Basin, N.S
An examination of the population characteristics, movement patterns, and recreational fishing of striped bass (Morone saxatilis) in Minas Basin, N.S. during summer 2008 Report prepared for Minas Basin Pulp and Power Co. Ltd. Contributors: Jeremy E. Broome, Anna M. Redden, Michael J. Dadswell, Don Stewart and Karen Vaudry Acadia Center for Estuarine Research Acadia University Wolfville, NS B4P 2R6 June 2009 2 Executive Summary This striped bass study was initiated because of the known presence of both Shubenacadie River origin and migrant USA striped bass in the Minas Basin, the “threatened” species COSEWIC designation, the existence of a strong recreational fishery, and the potential for impacts on the population due to the operation of in- stream tidal energy technology in the area. Striped bass were sampled from Minas Basin through angling creel census during summer 2008. In total, 574 striped bass were sampled for length, weight, scales, and tissue. In addition, 529 were tagged with individually numbered spaghetti tags. Striped bass ranged in length from 20.7-90.6cm FL, with a mean fork length of 40.5cm. Data from FL(cm) and Wt(Kg) measurements determined a weight-length relationship: LOG(Wt) = 3.30LOG(FL)-5.58. Age frequency showed a range from 1-11 years. The mean age was 4.3 years, with 75% of bass sampled being within the Age 2-4 year class. Total mortality (Z) was estimated to be 0.60. Angling effort totalling 1732 rod hours was recorded from June to October, 2008, with an average 7 anglers fishing per tide. Catch per unit effort (Fish/Rod Hour) was determined to be 0.35, with peak landing periods indicating a relationship with the lunar cycle. -

Fishing the Gaspereau Chris Gertridge
Wolfville Historical Society Summer 2019 Newsletter – Regatta Edition Volume 19 Issue 2 Up Tails All! An Account of the Inaugural Randall House Regatta By Martin Hallett, President Having dodged a meteorological bullet on Canada Day, when it looked as if the WHS hotdog stand in Willow Park would be submerged for the second time in three years, our merry band of BBQers could be forgiven for feeling that the weather gods Just don’t like the Historical Society – the only consolation being that if anyone fell into the pond, they couldn’t get Photo by C. Gertridge any wetter. problem by sending in their renowned team of hotdoggers But wouldn’t you know it -- we to feed the communal tummy; those in need of some dodged another bullet! The rain held off, sweetness in their lives were quick to spot The Real Scoop more or less, and by the time we packed up, Ice-cream stand Just a hop, skip and a Jump away. And a rather steamy sun was making its presence those who wished to offer their faces for artistic purposes felt. were obliged by Mme. Jasmine Renaud, who moonlights We really didn’t know what to as a WHS board-member. expect: the sale of boats had gone well, but Last but not least, the Joys of “messing about in whether that would translate into a host of boats” (or, in our case, “with”) were attested to by young would-be sailors eager to commit Michael Bawtree, who read – as only he can – an their craft to the deeps of Willow Pond appropriate chapter from Kenneth Grahame’s The Wind remained to be seen. -
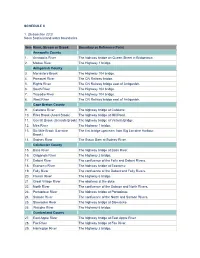
Nova Scotia Inland Water Boundaries Item River, Stream Or Brook
SCHEDULE II 1. (Subsection 2(1)) Nova Scotia inland water boundaries Item River, Stream or Brook Boundary or Reference Point Annapolis County 1. Annapolis River The highway bridge on Queen Street in Bridgetown. 2. Moose River The Highway 1 bridge. Antigonish County 3. Monastery Brook The Highway 104 bridge. 4. Pomquet River The CN Railway bridge. 5. Rights River The CN Railway bridge east of Antigonish. 6. South River The Highway 104 bridge. 7. Tracadie River The Highway 104 bridge. 8. West River The CN Railway bridge east of Antigonish. Cape Breton County 9. Catalone River The highway bridge at Catalone. 10. Fifes Brook (Aconi Brook) The highway bridge at Mill Pond. 11. Gerratt Brook (Gerards Brook) The highway bridge at Victoria Bridge. 12. Mira River The Highway 1 bridge. 13. Six Mile Brook (Lorraine The first bridge upstream from Big Lorraine Harbour. Brook) 14. Sydney River The Sysco Dam at Sydney River. Colchester County 15. Bass River The highway bridge at Bass River. 16. Chiganois River The Highway 2 bridge. 17. Debert River The confluence of the Folly and Debert Rivers. 18. Economy River The highway bridge at Economy. 19. Folly River The confluence of the Debert and Folly Rivers. 20. French River The Highway 6 bridge. 21. Great Village River The aboiteau at the dyke. 22. North River The confluence of the Salmon and North Rivers. 23. Portapique River The highway bridge at Portapique. 24. Salmon River The confluence of the North and Salmon Rivers. 25. Stewiacke River The highway bridge at Stewiacke. 26. Waughs River The Highway 6 bridge. -

Atlantic Maritime Ecozone+: Evidence for Key Findings
Atlantic Maritime Ecozone+ evidence for key findings summary Canadian Biodiversity: Ecosystem Status and Trends 2010 Evidence for Key Findings Summary Report No. 3 Published by the Canadian Councils of Resource Ministers Library and Archives Canada Cataloguing in Publication Atlantic Maritime Ecozone+ evidence for key findings summary. Issued also in French under title: Sommaire des éléments probants relativement aux constatations clés pour l’écozone+ maritime de l’Atlantique. Electronic monograph in PDF format. En14-43/0-3-2015E-PDF 978-1-100-23826-5 Information contained in this publication or product may be reproduced, in part or in whole, and by any means, for personal or public non-commercial purposes, without charge or further permission, unless otherwise specified. You are asked to: Exercise due diligence in ensuring the accuracy of the materials reproduced; Indicate both the complete title of the materials reproduced, as well as the author organization; and Indicate that the reproduction is a copy of an official work that is published by the Government of Canada and that the reproduction has not been produced in affiliation with or with the endorsement of the Government of Canada. Commercial reproduction and distribution is prohibited except with written permission from the author. For more information, please contact Environment Canada’s Inquiry Centre at 1- 800-668-6767 (in Canada only) or 819-997-2800 or email to [email protected]. Cover photos: Margaree Valley, Cape Breton Island, Nova Scotia, © iStock.com / cworthy; Hopewell Rocks, Bay of Fundy, New Brunswick, © iStock.com / MorganLeFaye This report should be cited as: ESTR Secretariat. 2014. -

They Planted Well: New England Planters in Maritime Canada
They Planted Well: New England Planters in Maritime Canada. PLACES Acadia University, Wolfville, Nova Scotia, 9, 10, 12 Amherst Township, Nova Scotia, 124 Amherst, Nova Scotia, 38, 39, 304, 316 Andover, Maryland 65 Annapolis River, Nova Scotia, 22 Annapolis Township, Nova Scotia, 23, 122-123 Annapolis Valley, Nova Scotia, 10, 14-15, 107, 178 Annapolis County, Nova Scotia, 20, 24-26, 28-29, 155, 258 Annapolis Gut, Nova Scotia, 43 Annapolis Basin, Nova Scotia, 25 Annapolis-Royal (Port Royal-Annapolis), 36, 46, 103, 244, 251, 298 Atwell House, King's County, Nova Scotia, 253, 258-259 Aulac River, New Brunswick, 38 Avon River, Nova Scotia, 21, 27 Baie Verte, Fort, (Fort Lawrence) New Brunswick, 38 Barrington Township, Nova Scotia, 124, 168, 299, 315, Beaubassin, New Brunswick (Cumberland Basin), 36 Beausejour, Fort, (Fort Cumberland) New Brunswick, 17, 22, 36-37, 45, 154, 264, 277, 281 Beaver River, Nova Scotia, 197 Bedford Basin, Nova Scotia, 100 Belleisle, Annapolis County, Nova Scotia, 313 Biggs House, Gaspreau, Nova Scotia, 244-245 Blomidon, Cape, Nova Scotia, 21, 27 Boston, Massachusetts, 18, 30-31, 50, 66, 69, 76, 78, 81-82, 84, 86, 89, 99, 121, 141, 172, 176, 215, 265 Boudreau's Bank, (Starr's Point) Nova Scotia, 27 Bridgetown, Nova Scotia, 196, 316 Buckram (Ship), 48 Bucks Harbor, Maine, 174 Burton, New Brunswick, 33 Calkin House, Kings County, 250, 252, 259 Camphill (Rout), 43-45, 48, 52 Canning, Nova Scotia, 236, 240 Canso, Nova Scotia, 23 Cape Breton, Nova Scotia, 40, 114, 119, 134, 138, 140, 143-144 2 Cape Cod-Style House, 223 -

Support for Delineation of Inner Bay of Fundy Salmon Marine Critical Habitat Boundaries in Minas Basin and Chignecto
Canadian Science Advisory Secretariat Maritimes Region Science Response 2015/035 SUPPORT FOR DELINEATION OF INNER BAY OF FUNDY SALMON MARINE CRITICAL HABITAT BOUNDARIES IN MINAS BASIN AND CHIGNECTO BAY Context In April 2014, the Fisheries and Oceans Canada (DFO) Species at Risk Management Division (SARMD) in the Maritimes Region requested information from DFO Science to assist with the delineation of boundaries for critical habitat (CH) being considered for Inner Bay of Fundy (IBOF) Atlantic Salmon within Chignecto Bay and Minas Basin, specifically: to assist with the delineation of the boundary between estuarine and marine habitat for several large, tidal estuaries (i.e., Petitcodiac River, Avon River, Salmon River Colchester, Shubenacadie River estuary and Cumberland Basin). DFO Science had previously provided advice on the characteristics and general location of important marine and estuarine habitat for IBOF salmon (DFO 2008; DFO 2013); however, additional information was requested to assist in delineating the precise boundaries of important marine habitat within Chignecto Bay and Minas Basin in order to subsequently propose, describe and map these as CH within an amended Recovery Strategy for IBOF salmon. Once identified in the Recovery Strategy, measures will be taken to protect this marine CH under the Species at Risk Act (SARA). This Science Response Report results from the Science Response Process of 11 July 2014 on Support for Delineation of Inner Bay of Fundy Salmon Marine Critical Habitat Boundaries. Background The inner Bay of Fundy populations of Atlantic salmon (Salmo salar) are listed as Endangered under the Species at Risk Act, and SARA requires the identification of CH for endangered species within a Recovery Strategy (or Action Plan). -
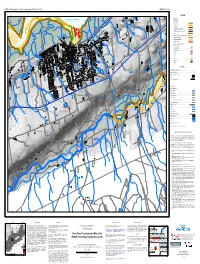
Open File Map ME 2012-009: Shore Zone Characterization Map of The
NSTDB 1:10 000 map sheet 10 450500 64300 (part of NTS sheet 21H/01) 64°24'0"W OFM ME 2012-009 N 390000mE 45°6'0"N 91 92 000m 93 95 94 49 (( 95 ( 96 64°18'0"W ( 397000mE (( * ( " " S" ( " 45°6'0"N LEGEND " " " ( " SOUTHERN BIGHT-MINAS BASIN RAMSAR SITE ( " ( " ( " " " ( " ( " " Minas Basin " " ( ( Shore Zones (( (( S" " " Backshore ( " ( ( S" ( " LOWER WOLFVILLE ( ( ( " ( ( " Highly stable . r ( ( " ( ( " " " e " ( " ( ( " " v ( i ( ( " ( ( " " Partially stable . R ( ( ( " " ( ( ( ( " " " s ( ( ( " i ( ( " " l ( ( ( l Not stable . ( " " " S" ( " a ( ( " ( ( ( " " " ( ( w S"( ( " ( ( Unconsolidated over bedrock . n ( ( ( r S" ( ( " ( ( " ( ( o " ( ( ( ( ( " C Other ( " (bulkhead,causeway, road, wharf). ( ( " ( ( ( ( " " ( " " Dyke . ( ( ( " " ( ( ( " " " " ( ( " ( ( " ( ( ( ( " " Foreshore ( " " " (upper foreshore, middle foreshore, lower foreshore) ( ( " " ( ( " ( ( ( ( ( Cliffed, highly stable . ( " " " " ( ( ( " ( " " " ( ( ( " ( ( Cliffed, partially stable . ((( " " " ( ( ( ( " " ( " " " ( " " " " (( ( ( " ( ( ( " ( ( ( U " Cliffed, not stable . ( ( ( ( " ( " ( " " " ( ( " ( ( ( ( ( " " Foreshore and Nearshore Habitats ( ( ( " " " " " ( ( " " 1 " " " N High salt marsh . " 94 ( ( " " " S " " " 000m ( ( " " " " Low salt marsh . " " " " 94 " " " ( ( " 49 ( ( " ( " " " " " Restored marsh . " " " " " " " " " " " " " " " " "" " " " " " " " " Cobble . " " " " " " " " " " " " WOLFVILLE " " Gravel. " " " " " " " " " " -

Gaspereau Maritimes Region Overview Limits, Are Implemented
Fisheries Pêches and Oceans et Océans DFO Science Maritimes Region Stock Status Report D3-17 Species proportion Blueback herring Alewife Bon Harriott Leim & Scott 1966 Gaspereau Southern Gulf Maritimes Region Overview Background Bay of Fundy Alewife (Alosa pseudoharengus) and blueback herring Nova Scotia Coast (Alosa aestivalis) are anadromous clupeids that frequent the rivers of the Maritimes. They are collectively referred to as gaspereau. Blueback herring occur in fewer rivers and are generally less abundant than alewives where both species co-occur. Spawning migrations of alewives typically begin in late April or early May, depending upon The Fishery geographic area and water temperature, peak in late May or early June and are completed by late June or early Landings (t) July. Blueback herring enter the river about 2 weeks later Year 70-79 80-89 1992 1993 1994 1995 1996 than do alewives. Both species return to sea soon after Avg. Avg. spawning. Young-of-the-year gaspereau spend, at most, S.Gulf 3704 4848 4544 4722 3806 3452 2150 the first summer and fall in fresh water before migrating NS.Coast 1279 893 497 803 973 1439 1365 to the sea. Both species recruit to the spawning stock over B. of F. 4184 1836 1618 1137 863 1230 1275 2-4 years. Spawning occurs first in both species at age 3 and virtually all fish have spawned by age 6. The mean TOTAL 9167 7578 6659 6662 5642 6120 4790 age at first spawning is usually older for females than for males. Repeat spawners may form a high proportion (35- 90%) of the stocks of both species, with higher The gaspereau fisheries are regulated by proportions of repeat spawners where exploitation is low. -

Plymouth Gentian,Sabatia Kennedyana
COSEWIC Assessment and Status Report on the Plymouth Gentian Sabatia kennedyana in Canada ENDANGERED 2012 COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: COSEWIC. 2012. COSEWIC assessment and status report on the Plymouth Gentian Sabatia kennedyana in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xi + 46 pp. (www.registrelep-sararegistry.gc.ca/default_e.cfm). Previous report(s): COSEWIC. 2000. COSEWIC assessment and update status report on the Plymouth gentian Sabatia kennedyana in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vii + 10 pp. Newell, R.E. 1999. Update COSEWIC status report on the Plymouth gentian Sabatia kennedyana in Canada, in COSEWIC assessment and update status report on the Plymouth gentian Sabatia kennedyana in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 1-10 pp. Keddy, C., and P. Keddy. 1984. COSEWIC status report on the Plymouth gentian Sabatia kennedyana in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 27 pp. Production note: COSEWIC would like to acknowledge Sean Blaney and Nicholas Hill for writing the status report on Plymouth Gentian, Sabatia kennedyana, in Canada, prepared under contract with Environment Canada. This report was overseen and edited by Bruce Bennett, Co-chair of the COSEWIC Vascular Plants Specialist Subcommittee. For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment Canada Ottawa, ON K1A 0H3 Tel.: 819-953-3215 Fax: 819-994-3684 E-mail: COSEWIC/[email protected] http://www.cosewic.gc.ca Également disponible en français sous le titre Ếvaluation et Rapport de situation du COSEPAC sur la Sabatie de Kennedy (Sabatia kennedyana) au Canada.