American Chestnut, Castanea Dentata
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CHESTNUT (CASTANEA Spp.) CULTIVAR EVALUATION for COMMERCIAL CHESTNUT PRODUCTION
CHESTNUT (CASTANEA spp.) CULTIVAR EVALUATION FOR COMMERCIAL CHESTNUT PRODUCTION IN HAMILTON COUNTY, TENNESSEE By Ana Maria Metaxas Approved: James Hill Craddock Jennifer Boyd Professor of Biological Sciences Assistant Professor of Biological and Environmental Sciences (Director of Thesis) (Committee Member) Gregory Reighard Jeffery Elwell Professor of Horticulture Dean, College of Arts and Sciences (Committee Member) A. Jerald Ainsworth Dean of the Graduate School CHESTNUT (CASTANEA spp.) CULTIVAR EVALUATION FOR COMMERCIAL CHESTNUT PRODUCTION IN HAMILTON COUNTY, TENNESSEE by Ana Maria Metaxas A Thesis Submitted to the Faculty of the University of Tennessee at Chattanooga in Partial Fulfillment of the Requirements for the Degree of Master of Science in Environmental Science May 2013 ii ABSTRACT Chestnut cultivars were evaluated for their commercial applicability under the environmental conditions in Hamilton County, TN at 35°13ꞌ 45ꞌꞌ N 85° 00ꞌ 03.97ꞌꞌ W elevation 230 meters. In 2003 and 2004, 534 trees were planted, representing 64 different cultivars, varieties, and species. Twenty trees from each of 20 different cultivars were planted as five-tree plots in a randomized complete block design in four blocks of 100 trees each, amounting to 400 trees. The remaining 44 chestnut cultivars, varieties, and species served as a germplasm collection. These were planted in guard rows surrounding the four blocks in completely randomized, single-tree plots. In the analysis, we investigated our collection predominantly with the aim to: 1) discover the degree of acclimation of grower- recommended cultivars to southeastern Tennessee climatic conditions and 2) ascertain the cultivars’ ability to survive in the area with Cryphonectria parasitica and other chestnut diseases and pests present. -

Cryphonectria Parasitica Global Invasive Species Database (GISD)
FULL ACCOUNT FOR: Cryphonectria parasitica Cryphonectria parasitica System: Terrestrial Kingdom Phylum Class Order Family Fungi Ascomycota Sordariomycetes Diaporthales Valsaceae Common name Edelkastanienkrebs (German), chestnut blight (English) Synonym Endothia parasitica Similar species Cryphonectria radicalis, Endothia gyrosa Summary Cryphonectria parasitica is a fungus that attacks primarily Castanea spp. but also has been known to cause damage to various Quercus spp. along with other species of hardwood trees. American chestnut, C. dentata, was a dominant overstorey species in United States forests, but now they have been completely replaced within the ecosystem. C. dentata still exists in the forests but only within the understorey as sprout shoots from the root system of chestnuts killed by the blight years ago. A virus that attacks this fungus appears to be the best hope for the future of Castanea spp., and current research is focused primarily on this virus and variants of it for biological control. Chestnut blight only infects the above-ground parts of trees, causing cankers that enlarge, girdle and kill branches and trunks. view this species on IUCN Red List Global Invasive Species Database (GISD) 2021. Species profile Cryphonectria Pag. 1 parasitica. Available from: http://www.iucngisd.org/gisd/species.php?sc=124 [Accessed 05 October 2021] FULL ACCOUNT FOR: Cryphonectria parasitica Species Description The US Forest Service (undated) states that, \"C. parasitica forms yellowish or orange fruiting bodies (pycnidia) about the size of a pin head on the older portion of cankers. Spores may exude from the pycnidia as orange, curled horns during moist weather. Stem cankers are either swollen or sunken, and the sunken type may be grown over with bark. -

LVL - Laminated Veneer Lumber) by Pollmeier Health Product by Pollmeier Inc
BauBuche (LVL - Laminated Veneer Lumber) by Pollmeier Health Product by Pollmeier Inc. Declaration v2.2 created via: HPDC Online Builder HPD UNIQUE IDENTIFIER: 20788 CLASSIFICATION: 06 71 13 Wood and Plastic PRODUCT DESCRIPTION: BauBuche LVL by Pollmeier - Made from 100% sustainable PEFC and FSC certified European Beech lumber and ULEF resorcinol resins. BauBuche LVL panels can be used for a variety of non structural architectural applications like flooring, wall paneling, table tops, furniture, mouldings, and various millwork projects. Section 1: Summary Basic Method / Product Threshold CONTENT INVENTORY Inventory Reporting Format Threshold level Residuals/Impurities All Substances Above the Threshold Indicated Are: Nested Materials Method 100 ppm Considered Characterized Yes Ex/SC Yes No Basic Method 1,000 ppm Partially Considered % weight and role provided for all substances. Per GHS SDS Not Considered Threshold Disclosed Per Other Explanation(s) provided Screened Yes Ex/SC Yes No Material for Residuals/Impurities? All substances screened using Priority Hazard Lists with Product Yes No results disclosed. Identified Yes Ex/SC Yes No One or more substances not disclosed by Name (Specific or Generic) and Identifier and/ or one or more Special Condition did not follow guidance. CONTENT IN DESCENDING ORDER OF QUANTITY Number of Greenscreen BM-4/BM3 contents ... 0 Summary of product contents and results from screening individual chemical Contents highest concern GreenScreen substances against HPD Priority Hazard Lists and the GreenScreen for Safer Benchmark or List translator Score ... LT-P1 Chemicals®. The HPD does not assess whether using or handling this Nanomaterial ... No product will expose individuals to its chemical substances or any health risk. -

Frank Meyer, Isabel Shipley Cunningham Agricultural Explorer
Frank Meyer, Isabel Shipley Cunningham Agricultural Explorer For 60 years the work of Frank N. Meyer has 2,500 pages of his letters tell of his journeys remained a neglected segment of America’s and the plants he collected, and the USDA heritage. Now, as people are becoming con- Inventory of Seeds and Plants Imported con- cerned about feeding the world’s growing tains descriptions of his introductions. population and about the loss of genetic di- Until recently little was known about the versity of crops, Meyer’s accomplishments first 25 years of Meyer’s life, when he lived have a special relevance. Entering China in in Amsterdam and was called Frans Meijer. 1905, near the dawn of the single era when Dutch sources reveal that he was bom into a explorers could travel freely there, he be- loving family in 1875. Frans was a quiet boy, came the first plant hunter to represent a who enjoyed taking long walks, reading government and to search primarily for eco- about distant lands, and working in his fami- nomically useful plants rather than orna- ly’s small garden. By the time he had mentals. No one before him had spent 10 fimshed elementary school, he knew that ’ years crossing the mountains, deserts, farms, wanted to be a world traveler who studied he ’,if and forests of Asia in search of fruits, nuts, plants; however, his parents could not afford vegetables, grains, and fodder crops; no one to give him further education. When he was has done so since. 14 years old, he found work as a gardener’s During four plant-hunting expeditions to helper at the Amsterdam Botanical Garden. -

Castanea Mollissima Blume): the Roots of Nut Tree Domestication
ORIGINAL RESEARCH published: 25 June 2018 doi: 10.3389/fpls.2018.00810 Signatures of Selection in the Genomes of Chinese Chestnut (Castanea mollissima Blume): The Roots of Nut Tree Domestication Nicholas R. LaBonte 1*, Peng Zhao 2 and Keith Woeste 3 1 Department of Crop Sciences, University of Illinois Urbana-Champaign, Urbana, IL, United States, 2 Key Laboratory of Resource Biology and Biotechnology in Western China, Ministry of Education, College of Life Sciences, Northwest University, Xi’an, China, 3 Hardwood Tree Improvement and Regeneration Center, Northern Research Station, USDA Forest Service, West Lafayette, IN, United States Chestnuts (Castanea) are major nut crops in East Asia and southern Europe, and are unique among temperate nut crops in that the harvested seeds are starchy rather than oily. Chestnut species have been cultivated for three millennia or more in China, so it is likely that artificial selection has affected the genome of orchard-grown chestnuts. The genetics of Chinese chestnut (Castanea mollissima Blume) domestication are also of Edited by: interest to breeders of hybrid American chestnut, especially if the low-growing, branching S. Hong Lee, habit of Chinese chestnut, an impediment to American chestnut restoration, is partly University of South Australia, Australia the result of artificial selection. We resequenced genomes of wild and orchard-derived Reviewed by: Guo-Bo Chen, Chinese chestnuts and identified selective sweeps based on pooled whole-genome SNP Zhejiang Provincial People’s Hospital, datasets. We present candidate gene loci for chestnut domestication and discuss the China potential phenotypic effects of candidate loci, some of which may be useful genes for Chaeyoung Lee, Soongsil University, South Korea chestnut improvement in Asia and North America. -

Quercus Robur (Fagaceae)
Quercus robur (Fagaceae) The map description EEBIO area The integrated map shows the distribution and changes in the areal’s boundaries of pedunculate oak (Quercus robur). Q. robur is the dominant forest- formative species in the belt of broadleaf and mixed 4 needleleaf-broadleaf forests in the plains of the European part of the former USSR (Sokolov et al. 1977). In the northern part of its areal Q. robur grows in river valleys. In the central part, it forms mixed forests with Picea abies; closer to the south – a belt of broadleaf forests where Q. robur dominates. At the areal’s south boundary it forms small (marginal) forests in ravines and flood-plains (Atlas of Areals and Resources… , 1976). Q. robur belongs to the thermophilic species. The low temperature bound of possible occurrence of oak forests is marked by an average annual of 2?C l (http://www.forest.ru – in Russian). Therefore, l l l l l l hypotretically, oak areal boundaries will shift along l ll l l l l l l l with the changes in the average annual temperature. l l l l l l l l l l l For Yearly map of averaged mean annual air l l l l l l l l l l l l l l l l l l lll temperature (Afonin A., Lipiyaynen K., Tsepelev V., l l l l l l l ll ll l l l 2005) see http://www.agroatlas.spb.ru Climate. l l l l l l l l l l l l l l l l Oak forests are of great importance for the water l l l l l ll l lll l l l l l l l l l l l l l l l l l l l regime and soil structure, especially on the steep l l l l l l l l l l l l l l l l l l l l l l l l l l l l l slopes of river valleys and in forest-poor areas. -

Chestnut Growers' Guide to Site Selection and Environmental Stress
This idyllic orchard has benefited from good soil and irrigation. Photo by Tom Saielli Chestnut Growers’ Guide to Site Selection and Environmental Stress By Elsa Youngsteadt American chestnuts are tough, efficient trees that can reward their growers with several feet of growth per year. They’ll survive and even thrive under a range of conditions, but there are a few deal breakers that guarantee sickly, slow-growing trees. This guide, intended for backyard and small-orchard growers, will help you avoid these fatal mistakes and choose planting sites that will support strong, healthy trees. You’ll know you’ve done well when your chestnuts are still thriving a few years after planting. By then, they’ll be strong enough to withstand many stresses, from drought to a caterpillar outbreak, with much less human help. Soil Soil type is the absolute, number-one consideration when deciding where—or whether—to plant American chestnuts. These trees demand well-drained, acidic soil with a sandy to loamy texture. Permanently wet, basic, or clay soils are out of the question. So spend some time getting to know your dirt before launching a chestnut project. Dig it up, roll it between your fingers, and send in a sample for a soil test. Free tests are available through most state extension programs, and anyone can send a sample to the Penn State Agricultural Analytical Services Lab (which TACF uses) for a small fee. More information can be found at http://agsci.psu.edu/aasl/soil-testing. There are several key factors to look for. The two-foot-long taproot on this four- Acidity year-old root system could not have The ideal pH for American chestnut is 5.5, with an acceptable range developed in shallow soils, suggesting from about 4.5 to 6.5. -

5 Fagaceae Trees
CHAPTER 5 5 Fagaceae Trees Antoine Kremerl, Manuela Casasoli2,Teresa ~arreneche~,Catherine Bod6n2s1, Paul Sisco4,Thomas ~ubisiak~,Marta Scalfi6, Stefano Leonardi6,Erica ~akker~,Joukje ~uiteveld', Jeanne ~omero-Seversong, Kathiravetpillai Arumuganathanlo, Jeremy ~eror~',Caroline scotti-~aintagne", Guy Roussell, Maria Evangelista Bertocchil, Christian kxerl2,Ilga porth13, Fred ~ebard'~,Catherine clark15, John carlson16, Christophe Plomionl, Hans-Peter Koelewijn8, and Fiorella villani17 UMR Biodiversiti Genes & Communautis, INRA, 69 Route d'Arcachon, 33612 Cestas, France, e-mail: [email protected] Dipartimento di Biologia Vegetale, Universita "La Sapienza", Piazza A. Moro 5,00185 Rome, Italy Unite de Recherche sur les Especes Fruitikres et la Vigne, INRA, 71 Avenue Edouard Bourlaux, 33883 Villenave d'Ornon, France The American Chestnut Foundation, One Oak Plaza, Suite 308 Asheville, NC 28801, USA Southern Institute of Forest Genetics, USDA-Forest Service, 23332 Highway 67, Saucier, MS 39574-9344, USA Dipartimento di Scienze Ambientali, Universitk di Parma, Parco Area delle Scienze 1lIA, 43100 Parma, Italy Department of Ecology and Evolution, University of Chicago, 5801 South Ellis Avenue, Chicago, IL 60637, USA Alterra Wageningen UR, Centre for Ecosystem Studies, P.O. Box 47,6700 AA Wageningen, The Netherlands Department of Biological Sciences, University of Notre Dame, Notre Dame, IN 46556, USA lo Flow Cytometry and Imaging Core Laboratory, Benaroya Research Institute at Virginia Mason, 1201 Ninth Avenue, Seattle, WA 98101, -
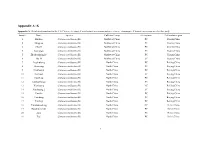
Appendix A~K
Appendix A~K Appendix A. Detailed information for the 146 Chinese chestnut (C.mollissima) accessions and nine chinese chinquapin (C.henryi) accessions used in this study Sample Name Species Cultivars Group Germplasm Cultivation region 1 Huijian Castanea mollissima Bl. Northwest China PC Shanxi,China 2 Mingjian Castanea mollissima Bl. Northwest China PC Shanxi,China 3 Chunli Castanea mollissima Bl. Northwest China PC Shanxi,China 4 Zuohongli Castanea mollissima Bl. Northwest China PC Shanxi,China 5 Zhenbashuangjie Castanea mollissima Bl. Northwest China PC Shanxi,China 6 zha 18 Castanea mollissima Bl. Northwest China LC Shanxi,China 7 Jingshuhong Castanea mollissima Bl. North China PC Beijing,China 8 Huaixiang Castanea mollissima Bl. North China PC Beijing,China 9 Huaihuang Castanea mollissima Bl. North China PC Beijing,China 10 Huzhaoli Castanea mollissima Bl. North China LC Beijing,China 11 Huaifeng Castanea mollissima Bl. North China PC Beijing,China 12 Yanshanhongli Castanea mollissima Bl. North China PC Beijing,China 13 Xiazhuang Castanea mollissima Bl. North China LC Beijing,China 14 Xinzhuang 2 Castanea mollissima Bl. North China LC Beijing,China 15 Huaijiu Castanea mollissima Bl. North China PC Beijing,China 16 Yanchang Castanea mollissima Bl. North China PC Beijing,China 17 Yanfeng Castanea mollissima Bl. North China PC Beijing,China 18 Yanshanzaofeng Castanea mollissima Bl. North China NC Hebei,China 19 Donglimingzhu Castanea mollissima Bl. North China PC Hebei,China 20 Zipo Castanea mollissima Bl. North China PC Hebei,China 21 Tasi Castanea mollissima Bl. North China PC Hebei,China 1 22 Zundali Castanea mollissima Bl. North China PC Hebei,China 23 Duanzhiyabian Castanea mollissima Bl. -

The Chestnut Blight Fungus for Studies on Virus/Host and Virus/Virus Interactions: from a Natural to a Model Host
Virology 477 (2015) 164–175 Contents lists available at ScienceDirect Virology journal homepage: www.elsevier.com/locate/yviro The chestnut blight fungus for studies on virus/host and virus/virus interactions: From a natural to a model host Ana Eusebio-Cope a, Liying Sun b, Toru Tanaka a, Sotaro Chiba a, Shin Kasahara c, Nobuhiro Suzuki a,n a Institute of Plant Science and Resources (IPSR), Okayama University, Chuou 2-20-1, Kurashiki, Okayama 710-0046, Japan b College of Plant Protection, Northwest A & F University, Yangling, Shananxi, China c Department of Environmental Sciences, Miyagi University, Sendai 982-215, Japan article info abstract Article history: The chestnut blight fungus, Cryphonectria parasitica, is an important plant pathogenic ascomycete. The Received 16 August 2014 fungus hosts a wide range of viruses and now has been established as a model filamentous fungus for Returned to author for revisions studying virus/host and virus/virus interactions. This is based on the development of methods for 15 September 2014 artificial virus introduction and elimination, host genome manipulability, available host genome Accepted 26 September 2014 sequence with annotations, host mutant strains, and molecular tools. Molecular tools include sub- Available online 4 November 2014 cellular distribution markers, gene expression reporters, and vectors with regulatable promoters that Keywords: have been long available for unicellular organisms, cultured cells, individuals of animals and plants, and Cryphonectria parasitica certain filamentous fungi. A comparison with other filamentous fungi such as Neurospora crassa has been Chestnut blight fungus made to establish clear advantages and disadvantages of C. parasitica as a virus host. In addition, a few dsRNA recent studies on RNA silencing vs. -

AMERICAN BEECH a Tree in Trouble
Hiawatha National Forest WARNING! While there are no pure beech stands on the AMERICAN Hiawatha National Forest (HNF), many of Wind in the Trees the hardwood stands include a significant BEECH component of beech. HNF has included The Forest Service is making every effort A Tree in Trouble these areas in the BBD Project Environmen- to identify and remove hazardous trees tal Analysis. These stands are within the ad- vancing front of the BBD and many of the from developed areas as quickly as possi- stands already have the beech scale present, ble. However, all visitors - but particularly particularly in the north and east portions of hikers and overnight backcountry campers the project area. Areas that have not yet been - should be alert for trees that are weak- infested will likely be infested within 1-3 ened, have large dead limbs or are com- years. Safety is the number one concern. Dy- ing beech trees will need to be removed in all pletely dead, especially in windy condi- high use public areas to prevent them from tions. becoming a safety hazard. The recreation Be alert. Look up. Choose your team on the HNF is assessing these areas campsites carefully. (including parking areas, campsites, trails, and The American beech (Fagus grandifolia) is a day use areas) to determine what the poten- tall, stately tree with smooth grey bark and tial hazards will be and the best way to deal Hiawatha National Forest a graceful arching crown. Its dark green, with them. Munising Ranger District shiny leaves, tapered at both ends, turn 400 East Munising Avenue golden in the fall and cling to its branches throughout the winter. -

Diversity of Wisconsin Rosids
Diversity of Wisconsin Rosids . oaks, birches, evening primroses . a major group of the woody plants (trees/shrubs) present at your sites The Wind Pollinated Trees • Alternate leaved tree families • Wind pollinated with ament/catkin inflorescences • Nut fruits = 1 seeded, unilocular, indehiscent (example - acorn) *Juglandaceae - walnut family Well known family containing walnuts, hickories, and pecans Only 7 genera and ca. 50 species worldwide, with only 2 genera and 4 species in Wisconsin Carya ovata Juglans cinera shagbark hickory Butternut, white walnut *Juglandaceae - walnut family Leaves pinnately compound, alternate (walnuts have smallest leaflets at tip) Leaves often aromatic from resinous peltate glands; allelopathic to other plants Carya ovata Juglans cinera shagbark hickory Butternut, white walnut *Juglandaceae - walnut family The chambered pith in center of young stems in Juglans (walnuts) separates it from un- chambered pith in Carya (hickories) Juglans regia English walnut *Juglandaceae - walnut family Trees are monoecious Wind pollinated Female flower Male inflorescence Juglans nigra Black walnut *Juglandaceae - walnut family Male flowers apetalous and arranged in pendulous (drooping) catkins or aments on last year’s woody growth Calyx small; each flower with a bract CA 3-6 CO 0 A 3-∞ G 0 Juglans cinera Butternut, white walnut *Juglandaceae - walnut family Female flowers apetalous and terminal Calyx cup-shaped and persistant; 2 stigma feathery; bracted CA (4) CO 0 A 0 G (2-3) Juglans cinera Juglans nigra Butternut, white