Spatial Variability in Growth and Prey Availability of Lobsters in the Northwestern Hawaiian Islands
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

American Samoa Archipelago Fishery Ecosystem Plan 2017
ANNUAL STOCK ASSESSMENT AND FISHERY EVALUATION REPORT: AMERICAN SAMOA ARCHIPELAGO FISHERY ECOSYSTEM PLAN 2017 Western Pacific Regional Fishery Management Council 1164 Bishop St., Suite 1400 Honolulu, HI 96813 PHONE: (808) 522-8220 FAX: (808) 522-8226 www.wpcouncil.org The ANNUAL STOCK ASSESSMENT AND FISHERY EVALUATION REPORT for the AMERICAN SAMOA ARCHIPELAGO FISHERY ECOSYSTEM PLAN 2017 was drafted by the Fishery Ecosystem Plan Team. This is a collaborative effort primarily between the Western Pacific Regional Fishery Management Council, NMFS-Pacific Island Fisheries Science Center, Pacific Islands Regional Office, Division of Aquatic Resources (HI) Department of Marine and Wildlife Resources (AS), Division of Aquatic and Wildlife Resources (Guam), and Division of Fish and Wildlife (CNMI). This report attempts to summarize annual fishery performance looking at trends in catch, effort and catch rates as well as provide a source document describing various projects and activities being undertaken on a local and federal level. The report also describes several ecosystem considerations including fish biomass estimates, biological indicators, protected species, habitat, climate change, and human dimensions. Information like marine spatial planning and best scientific information available for each fishery are described. This report provides a summary of annual catches relative to the Annual Catch Limits established by the Council in collaboration with the local fishery management agencies. Edited By: Marlowe Sabater, Asuka Ishizaki, Thomas Remington, and Sylvia Spalding, WPRFMC. This document can be cited as follows: WPRFMC, 2018. Annual Stock Assessment and Fishery Evaluation Report for the American Samoa Archipelago Fishery Ecosystem Plan 2017. Sabater, M., Ishizaki, A., Remington, T., Spalding, S. (Eds.) Western Pacific Regional Fishery Management Council. -

Emily Sue Stafford
Measuring and Interpreting Predation on Gastropod Shells by Emily Sue Stafford A thesis submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy Department of Earth and Atmospheric Sciences University of Alberta © Emily Sue Stafford, 2014 ABSTRACT This dissertation focuses on problems and progress in studying crushing predation on gastropods in the Modern and the fossil record. Although crushing predation tends to be destructive, it is possible to gather data on crushing predation from multiple angles. Chapter 2 applies an ichnotaxonomic name, Caedichnus, to the trace created by peeling crab predators. Chapter 3 the relationship between shell repair frequency and predation mortality in a modern gastropod community. In this case, repair frequency was likely a direct product of variation in predator abundance and strength. Chapter 4 focused on hermit crabs, an organism that inhabits gastropod shells and exposes those shells to predation even after the original gastropod inhabitant has died. The predatory crabs showed no preference for snail or hermit crab prey, which may mean that hermit crab habitation does not significantly alter the crab-on-snail predation patterns present in a shell assemblage. Chapter 5 expanded on previous work by the author, using a method by G.J. Vermeij to estimate crushing predation in a gastropod assemblage even when individual instances of predatory damage cannot be identified. Vermeij Crushing Analysis (VCA) uses drilled shells to establish a baseline of taphonomic damage in a shell assemblage; the chapter refines and examines this method more deeply, in addition to applying the method to compare predation on modern and fossil gastropod shell assemblages. -

Northwestern Hawaiian Islands Lobster Fishery
No. 9, October 2020 Northwestern Hawaiian Islands Lobster Fishery By Michael Markrich About the Author Michael Markrich is the former public information officer for the State of Hawai‘i Department of Land and Natural Resources; communications officer for State of Hawai‘i Department of Business, Economic Development and Tourism; columnists for the Honolulu Advertiser; socioeconomic analyst with John M. Knox and Associates; and consultant/owner of Markrich Research. He holds a bachelor of arts degree in history from the University of Washington and a master of science degree in agricultural and resource economics from the University of Hawai‘i. Disclaimer: The statements, findings and conclusions in this publication are those of the author and do not necessarily represent the views of the Western Pacific Regional Fishery Management Council or the National Marine Fisheries Service (NOAA). © Western Pacific Regional Fishery Management Council, 2020. All rights reserved, Published in the United States by the Western Pacific Regional Fishery Management Council under NOAA Award # NA20NMF4410013. ISBN 978-1-944827-73-1 Cover Art: NWHI lobster fishery. Photo: NOAA Contents List of Illustrations .................................................................................................................. i List of Tables .......................................................................................................................... i Acknowledgments ................................................................................................................ -

Reproductive Strategies Under Different Environmental Conditions: Total Abstract Output Vs Investment Per Egg in the Slipper Lobster Scyllarus Arctus
Journal of the Marine Reproductive strategies under different Biological Association of the United Kingdom environmental conditions: total output vs investment per egg in the slipper lobster cambridge.org/mbi Scyllarus arctus L. Fernández1 , C. García-Soler2 and I. Alborés1 Original Article 1Departamento de Biología, Facultad de Ciencias, Universidade da Coruña, Campus da Zapateira, 15071 A Coruña, 2 Cite this article: Fernández L, García-Soler C, Spain and Aquarium Finisterrae, Paseo Alcalde Francisco Vázquez, 15002 A Coruña, Spain Alborés I (2021). Reproductive strategies under different environmental conditions: total Abstract output vs investment per egg in the slipper lobster Scyllarus arctus. Journal of the Marine The slipper lobster Scyllarus arctus is an important fishery resource in Galicia (NW Iberian Biological Association of the United Kingdom Peninsula), with a large reduction of its populations in recent decades in the North-east – 101, 131 139. https://doi.org/10.1017/ Atlantic and Mediterranean, but only limited information on its reproduction. This study pro- S0025315421000035 vides an analysis of the reproductive potential of this scyllarid during two breeding cycles ′ ′ Received: 18 July 2020 (2008 and 2009) in the NE Atlantic (43°20 N 8°50 W). We studied several reproductive traits Revised: 5 January 2021 (fecundity, brood weight, egg weight and volume) in broods with eggs both in an early and Accepted: 7 January 2021 late embryonic stage, in relation to female size and temporal variations. Total output (fecund- First published online: 8 February 2021 ity and weight) and egg weight were closely linked to maternal size, and this relationship Key words: remained in broods with late-stage eggs. -

Decapoda: Scyllaridae)
Livro Vermelho dos Crustáceos do Brasil: Avaliação 2010-2014 ISBN 978-85-93003-00-4 © 2016 SBC CAPÍTULO 28 AVALIAÇÃO DAS LAGOSTAS-SAPATEIRAS (DECAPODA: SCYLLARIDAE) Luis Felipe A. Duarte, Allysson Pinheiro, William Santana, Evandro S. Rodrigues, Marcelo A. A. Pinheiro, Harry Boos & Petrônio A. Coelho (in memoriam) Palavras-chave: Achelata, ameaça, cavaquinha, extinção, impacto, Scyllarides. Introdução A família Scyllaridae (Latreille, 1825) é constituída por 85 espécies de lagostas, distribuídas em 20 gêneros e 4 subfamílias (Lavalli & Spanier, 2007), que se distinguem das demais lagostas por possuírem carapaça achatada, órbitas escavadas nas margens da superfície dorsal, antenas curtas, largas e escamiformes e um exoesqueleto muito espesso (Williams, 1965; Holthuis, 1995). Espécies dessa família, juntamente com as famílias Palinuridae, conhecidas popularmente como lagostas-verdadeiras ou espinhosas, e Sinaxidae, constituem a infraordem Achelata. Esse grupo compartilha várias características, entre elas a presença da larva filossoma, que é distintiva entre os Decapoda (Lavalli & Spanier, 2007). De maneira geral, embora os organismos dessa família não sejam alvo específico de pescarias ao redor do mundo, muitas espécies possuem valor comercial. Holthuis (1991) verificou que das 71 espécies conhecidas na época de seu estudo, 42,3% delas interessavam à atividade pesqueira, com destaque para: Scyllarides brasiliensis Rathbun, 1906; S. latus (Latreille, 1803); S. squamomosus (H. Milne Edwards, 1837); Ibacus alticrenatus Spence Bate, 1888; I. ciliatus (von Siebold, 1824); I. novemdentatus Gibbes, 1850; I. peronii Leach, 1815; Parribacus antarcticus (Lund, 1793); e Thenus orientalis (Lund, 1793). Spanier & Lavalli (2007) destacam a crescente importância que o gênero Scyllarides tende a ocupar nas pescarias. Em países de língua inglesa as espécies pertencentes à família Scyllaridae são conhecidas como slipper-lobster, spanish-lobster ou hooded-lobster. -

With Trachypenaeus Curvirostris (Sometimes Under the Name T
950 Shrimps and Prawns Trachypenaeus longipes (Paulson, 1875) En - Longlegged rough shrimp. Maximum body length 10.5 cm (females) and 8 cm (males). Found from nearshore waters to depths of about 220 m, usually between 40 and 60 m. Taken mainly by trawls. This species is often confused with Trachypenaeus curvirostris (sometimes under the name T. asper), and therefore its actual distribution and occurrence in the area is unclear. Probably not very common and of limited or no commercial importance. Indo-West Pacific from the Red Sea to Japan and the Philippines. distomedian distolateral projection anterior projection plate (after Motoh and Buri, 1984) posterior plate thelycum petasma (ventral view) (after Hayashi, 1992) Trachypenaeus sedili Hall, 1961 En - Singapore rough shrimp. (the FAO names previously used for this species are now used for Trachypenaeus malaiana) Maximum body length 8.8 cm (females) and 5.1 cm (males), commonly between 6 and 8 cm. Found on mud or sand bottom, from nearshore waters to depths of about 45 m.Taken by trawls and artisanal gear. Probably not a common species in the area and without commercial importance. Indo-West Pacific from India (perhaps Somalia) to the Malay Peninsula and South China Sea. anterior plate distomedian projection distolateral projection posterior plate thelycum petasma (ventral view) Penaeidae 951 Trachypenaeus villaluzi Muthu and Motoh, 1979 En - Philippines rough shrimp. Maximum body length 7.3 cm (females) and 5.3 cm (males). Caught by otter trawls at a depth of about 7 m, on mud bottom. Probably not common and without commercial importance. So far only known from the Philippines. -

Mediterranean Marine Science
Mediterranean Marine Science Vol. 21, 2020 Potential effects of elevated temperature on seasonal movements in slipper lobsters, Scyllarides latus (Latreille, 1803), in the eastern Mediterranean GOLDSTEIN JASON Wells National Estuarine Research Reserve, The Maine Coastal Ecology Center, 342 Laudholm Farm Road, Wells, ME, 04090 SPANIER EHUD Department of Maritime Civilizations, The Leon Recanati Institute for Maritime Studies & The Leon H. Charney School for Marine Sciences, University of Haifa, Mount Carmel, Haifa 3498838 https://doi.org/10.12681/mms.22074 Copyright © 2020 Mediterranean Marine Science To cite this article: GOLDSTEIN, J., & SPANIER, E. (2020). Potential effects of elevated temperature on seasonal movements in slipper lobsters, Scyllarides latus (Latreille, 1803), in the eastern Mediterranean. Mediterranean Marine Science, 21(2), 482-492. doi:https://doi.org/10.12681/mms.22074 http://epublishing.ekt.gr | e-Publisher: EKT | Downloaded at 14/07/2020 11:50:55 | Research Article Mediterranean Marine Science Indexed in WoS (Web of Science, ISI Thomson) and SCOPUS The journal is available on line at http://www.medit-mar-sc.net DOI: http://dx.doi.org/10.12681/mms.22074 Potential effects of elevated temperature on seasonal movements in slipper lobster, Scyllarides latus (Latreille, 1803), in the eastern Mediterranean Jason GOLDSTEIN1 and Ehud SPANIER2 1 Wells National Estuarine Research Reserve, The Maine Coastal Ecology Center, 342 Laudholm Farm Road, Wells, ME, 04090, USA 2 The Leon Recanati Institute for Maritime Studies & Department of Maritime Civilizations, The Leon H. Charney School for Marine Sciences, University of Haifa, Mount Carmel, Haifa 3498838, Israel Corresponding author: [email protected] Handling Editor: Stelios KATSANEVAKIS Received: 31 December 2019; Accepted: 15 June 2020; Published online: 13 July 2020 Abstract Temperature serves a predominant motivator for movement and activity over a wide range of mobile marine ectotherms. -

153 ANNOTATED Checklist of the World's Marine Lobsters
THE RAFFLES BULLETIN OF ZOOLOGY 2010 Supplement No. 23: 153–181 Date of Publication: 31 Oct.2010 © National University of Singapore ANNOTATED CHECKLIST OF THE WORLD’s MARINE LOBSTERS (CRUSTACEA: DECAPODA: ASTACIDEA, GLYPHEIDEA, ACHELATA, POLYCHELIDA) Tin-Yam Chan Institute of Marine Biology, National Taiwan Ocean University, Keelung 20224, Taiwan, R.O.C. Email:[email protected] ABSTRACT. – Marine lobsters are defined as consisting of four infraorders of decapod crustaceans: Astacidea, Glypheidea, Achelata and Polychelida. Together they form the suborder Macrura Reptantia. A checklist of the currently recognized six families, 55 genera and 248 species (with four subspecies) of living marine lobsters is provided, together with their synonyms in recent literature and information on the type locality of the valid taxa. Notes on alternative taxonomies and justifications for the choice of taxonomy are given. Although Caroli Linnaeus himself described the first marine lobster in 1758, the discovery rate of marine lobsters remains high to this day. KEY WORDS. – Crustacea, Decapoda, lobsters, marine, checklist, taxonomy. INTRODUCTION al., 2005). These results also showed that the relationships of the superfamilies and families of marine lobsters are mostly Commercialy, lobsters are generally the most highly prized different from the previously well-established scheme of crustaceans in all parts of the world. The taxonomy of marine Holthuis (1991). However, these phylogenetic studies have lobsters has remained fairly stable over many years and some yielded significantly contrasting results (see Patek et al., 2006, recent authors, such as Burukovsky (1983) and Phillips et al. Tsang et al., 2008; Bracken et al., 2009; Toon et al., 2009). -

Estimating Commercial Scallop Dredge Efficiency Through Vessel Tracking, Catch Data, and Depletion Models
W&M ScholarWorks Dissertations, Theses, and Masters Projects Theses, Dissertations, & Master Projects 2002 Estimating Commercial Scallop Dredge Efficiency through Vessel Tracking, Catch Data, and Depletion Models Todd Gedamke College of William and Mary - Virginia Institute of Marine Science Follow this and additional works at: https://scholarworks.wm.edu/etd Part of the Fresh Water Studies Commons, and the Oceanography Commons Recommended Citation Gedamke, Todd, "Estimating Commercial Scallop Dredge Efficiency through Vessel Tracking, Catch Data, and Depletion Models" (2002). Dissertations, Theses, and Masters Projects. Paper 1539617795. https://dx.doi.org/doi:10.25773/v5-xymz-rx92 This Thesis is brought to you for free and open access by the Theses, Dissertations, & Master Projects at W&M ScholarWorks. It has been accepted for inclusion in Dissertations, Theses, and Masters Projects by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. E s t im a t in g c o m m e r c ia l s c a l l o p d r e d g e e f f ic ie n c y t h r o u g h VESSEL TRACKING, CATCH DATA, AND DEPLETION MODELS A Thesis Presented to The Faculty of the School of Marine Science The College of William and Mary In Partial Fulfillment Of the Requirements for the Degree of Master of Science by Todd Gedamke 2002 APPROVAL SHEET This thesis is submitted in partial fulfillment of the requirements for the degree of Master of Science Todd Gedamke Approved, January 2002 William D. DuPaul Committee Chairman/Advisor d j - TEI Andrew J. -

A REVISION of the FAMILY SCYLLARIDAE (CRUSTACEA DECAPODA MACRURA). I. SUBFAMILY IBACINAE by L. B. HOLTHUIS CONTENTS 3
A REVISION OF THE FAMILY SCYLLARIDAE (CRUSTACEA DECAPODA MACRURA). I. SUBFAMILY IBACINAE by L. B. HOLTHUIS Holthuis, L. B.: A revision of the family Scyllaridae (Crustacea: Decapoda: Macrura). I. Sub- family Ibacinae. Zool. Verh. Leiden 218, 27-ii-1985: 1-130, figs. 1-27. — ISSN 0024-1652. Key words: Crustacea; Scyllaridae; key, subfamilies and genera; Ibacinae; Arctidinae; Theninae; Scyllarinae; Evibacus; Ibacus; Parribacus. General account of morphology and taxonomy of Scyllaridae with keys to subfamilies and genera. Establishment of subfamilies Ibacinae, Arctidinae, Theninae and Scyllarinae. Mono- graphic treatment of subfamily Ibacinae and its genera Evibacus (one species), Ibacus (six species, one subspecies), and Parribacus (six species). No new species. L. B. Holthuis, Rijksmuseum van Natuurlijke Historie, P.O. Box 9517, 2300 RA Leiden, The Netherlands. CONTENTS Introduction 4 Scyllaridae 4 Key to the genera 11 Ibacinae 12 Evibacus 13 E. princeps 14 Ibacus 21 Key to the species 22 I. c. ciliatus 24 I. c. pubescens 33 I. alticrenatus 36 I. brucei 41 I. brevipes 47 I. novemdentatus 52 I. peronii 61 Parribacus 69 Key to the species 71 P. antarcticus 73 P. caledonicus 88 P. perlatus 93 P. holthuisi 98 P. scarlatinus 102 P. japonicus 106 References 111 3 4 ZOOLOGISCHE VERHANDELINGEN 218 (1985) INTRODUCTION In 1960, at the invitation of the Smithsonian Institution, a study was under- taken of the rich Scyllarid collections of the United States National Museum, Washington, D.C. (USNM), with the object to revise this family of Crustacea. The studied material, in which most of the known species are represented, clearly showed the necessity of such a revision, and at the same time offered the materials for it. -
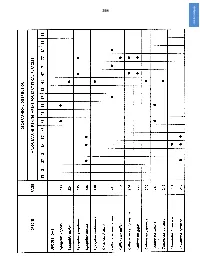
Click for Next Page Click for Previous Page 265
264 click for next page click for previous page 265 4. BIBLIOGRAPHY Albert, F., 1898. La langosta de Juan Fernandez i la posibilidad de su propagación en la costa Chilena. Revista Chilena Historia natural, 2:5-11, 17-23,29-31, 1 tab Alcock, A., 1901. A descriptive catalogue of the Indian deep-Sea Crustacea Decapoda Macrura and Anomala in the Indian Museum. Being a revised account of the deep-sea species collected by the Royal Indian Marine Survey Ship Investigator: 1-286, i-iv, pls 1-3 Alcock, A. & A.R.S. Anderson, 1896. Illustrations of the Zoology of the Royal Indian Marine Surveying Steamer Investigator, under the command of Commander CF. Oldham, R.N. Crustacea (4):pls 1-6-27 Alcock, A. & A.F. McArdle, 1902. Illustrations of the Zoology of the Royal Indian Marine Survey Ship Investigator, under the command of Captain T.H. Heming, R.N. Crustacea, (10), pls 56-67 Allsopp, W.H.L., 1968. Report to the government of British Honduras (Belize) on investigations into marine fishery management, research and development policy for Spiny Lobster fisheries. Report U.N. Development Program FAO, TA 2481 :i-xii, 1-86, figs 1-15 Arana Espina, P. & C.A. Melo Urrutia, 1973. Pesca comercial’de Jasus frontalis en las Islas Robinson Crusoe y Santa Clara. (1971-1972). La Langosta de Juan Fernández II. Investigaciones Marinas, Valparaiso, 4(5):135-152, figs 1-5. For no. I see next item, for III see Pizarro et al., 1974, for IV see Pavez Carrera et al., 1974 Arana Espina, P. -

Downloaded from Genbank for All Australian Palinurid And
ResearchOnline@JCU This is the author-created version of the following work: Woodings, Laura N., Murphy, Nicholas P., Jeffs, Andrew, Suthers, Iain M., Liggins, Geoffrey, and Strugnell, Jan M. (2019) Distribution of Palinuridae and Scyllaridae phyllosoma larvae within the East Australian Current: a climate change hot spot. Marine and Freshwater Research, 70 pp. 1020-1033. Access to this file is available from: https://researchonline.jcu.edu.au/61097/ Journal compilation © CSIRO 2019. The Accepted Manuscript version of this paper is available Open Access from ResearchOnline@JCU by permission of the publisher. Please refer to the original source for the final version of this work: https://doi.org/10.1071/MF18331 Title: Distribution of Palinuridae and Scyllaridae phyllosoma larvae within the East Australian Current: a climate change hot-spot Authors: Laura N. WoodingsA,G, Nick P. MurphyA, Andrew JeffsB, Iain M. SuthersC, Geoffrey W. LigginsD, Bridget S. GreenE, Jan M. StrugnellA,F. ADepartment of Ecology, Environment and Evolution, School of Life Sciences, La Trobe University, Bundoora, Vic. 3086, Australia. BSchool of Biological Sciences and Institute of Marine Science, University of Auckland, Auckland 1142, New Zealand. CSchool of Biological, Earth & Environmental Sciences, University of New South Wales, Sydney, NSW 2052, Australia. DNSW Department of Primary Industries, Sydney Institute of Marine Science, Mosman NSW 2088, Australia EInstitute for Marine and Antarctic Studies, University of Tasmania, Hobart, Tas. 7001, Australia FCentre for Sustainable Tropical Fisheries and Aquaculture and College of Science and Engineering, James Cook University, Townsville, Qld 4811, Australia. GCorresponding author: Email: [email protected] Abstract Many marine species are predicted to shift their ranges poleward due to rising ocean temperatures driven by climate change.