University of Valladolid
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Electronic Payment Systems for Competitive Advantage in E-Commerce Francisco Liebana-Cabanillas University of Granada, Spain
Electronic Payment Systems for Competitive Advantage in E-Commerce Francisco Liebana-Cabanillas University of Granada, Spain Francisco Munoz-Leiva University of Cranada, Spain Juan Sänchez-Fernändez University of Cranada, Spain Myriam Martinez-Fiestas £5/4/V University, Peru A volume in the Advances in E-Business BUSINESS SCIENCE Research (AEBR) Book Series Heferewce/ An Imprint of IGI Global Table of Contents Preface Acknowledgment Section 1 Internet as a Buy and Seil Distribution Channel Chapter 1 The Role of B2B E-Commerce in Market Share: Evidence from Spanish Manufacturing Firms Juliette Milgram-Baleix, University of Granada, Spain Melanie Parravano, University of East Anglia, UK Luis Enrique Pedauga, University of Granada, Spain Chapter 2 Internet as a Sales Channel for the Agri-Food Sector: A Case Study of Organic Products Enrique Bernal Jurado, University of Jaen, Spain Adoraciön Mozas Moral, University of Jaen, Spain Miguel J. Medina Viruel, University of Jaen, Spain Chapter 3 How to Develop WOM Marketing Manuela Lopez, University of Murcia, Spain Maria Sicilia, University of Murcia, Spain Section 2 Modelling of Users' Behaviour towards New Electronic Environments Chapter 4 New Market Segmentation Paradigms and Electronic Commerce Adoption: An Exploratory Study.. Angel E Agudo-Peregrina, Technical University of Madrid, Spain Julian Chaparro-Peläez, Technical University of Madrid, Spain Angel Hernändez-Garcia, Technical University of Madrid, Spain Chapter 5 How Can E-Vendors Create Trust in B2C and C2C Contexts? Sonia San-Martin, University of Burgos, Spain Carmen Camarero, University of Valladolid, Spain Chapter 6 Perceived Risk in E-Commerce and the Development of Loyalty: The Moderating Effect of Website Design, the Cultural Framework of Language, and the User's Flow State 93 Juan Miguel Alcäntara-Pilar, University of Granada, Spain Salvador del Barrio-Garcia, University of Granada, Spain Chapter 7 The Relationship Between E-WOM from SNS or Internet and Purchase 1 F. -

ERASMUS+ KA107-36589 – ICM – University of Valladolid
ERASMUS+ KA107-36589 – ICM – University of Valladolid This project is coordinated by the University of Valladolid, within the framework of the European Programme + Key Action 1 “International Credit Mobility”. It is a project of mobility of undergraduate, master and doctorate mobility, in addition to academic and administrative staff, between the University of Valladolid and the Partner Countries from the targeted countries. ERASMUS+ PROGRAMME: Erasmus+ is the EU Programme in the fields of education, training, youth and sport for the period 2014-2020. Projects under this Action promote transnational mobility activities targeting students and academic and administrative staff. PARTNER INSTITUTIONS: INTERNATIONAL RELATIONS SERVICE CASA DEL ESTUDIANTE, Real de Burgos s/n 47011 VALLADOLID This project has been funded with support from the European Commission. (Spain) This communication reflects the views only of the author, and the Commission cannot be held responsible for any use which may be made of the information contained Phone: +34 983 18 4785 – Fax: +34 983 42 3748 therein. E-mail: [email protected] - http://www.relint.uva.es/ Erasmus+ 2017-1-ES01-KA107-036589 ACRONYM Name Country AASTMT ARAB ACADEMY FOR SCIENCE AND TECHNOLOGY AND MARITIME TRANSPORT Egypt CU CAIRO UNIVERSITY Egypt UES UNIVERSIDAD DE EL SALVADOR El Salvador FNU FIJI NATIONAL UNIVERSITY Fiji USP THE UNIVERSITY OF THE SOUTH PACIFIC Fiji USTM UNIVERSITÉ DES SCIENCES ET TECHNIQUES DE MASUKU Gabon GTU GEORGIAN TECHNICAL UNIVERSITY Georgia SSOUT SULKHAN - SABA ORBELIANI -

College Codes (Outside the United States)
COLLEGE CODES (OUTSIDE THE UNITED STATES) ACT CODE COLLEGE NAME COUNTRY 7143 ARGENTINA UNIV OF MANAGEMENT ARGENTINA 7139 NATIONAL UNIVERSITY OF ENTRE RIOS ARGENTINA 6694 NATIONAL UNIVERSITY OF TUCUMAN ARGENTINA 7205 TECHNICAL INST OF BUENOS AIRES ARGENTINA 6673 UNIVERSIDAD DE BELGRANO ARGENTINA 6000 BALLARAT COLLEGE OF ADVANCED EDUCATION AUSTRALIA 7271 BOND UNIVERSITY AUSTRALIA 7122 CENTRAL QUEENSLAND UNIVERSITY AUSTRALIA 7334 CHARLES STURT UNIVERSITY AUSTRALIA 6610 CURTIN UNIVERSITY EXCHANGE PROG AUSTRALIA 6600 CURTIN UNIVERSITY OF TECHNOLOGY AUSTRALIA 7038 DEAKIN UNIVERSITY AUSTRALIA 6863 EDITH COWAN UNIVERSITY AUSTRALIA 7090 GRIFFITH UNIVERSITY AUSTRALIA 6901 LA TROBE UNIVERSITY AUSTRALIA 6001 MACQUARIE UNIVERSITY AUSTRALIA 6497 MELBOURNE COLLEGE OF ADV EDUCATION AUSTRALIA 6832 MONASH UNIVERSITY AUSTRALIA 7281 PERTH INST OF BUSINESS & TECH AUSTRALIA 6002 QUEENSLAND INSTITUTE OF TECH AUSTRALIA 6341 ROYAL MELBOURNE INST TECH EXCHANGE PROG AUSTRALIA 6537 ROYAL MELBOURNE INSTITUTE OF TECHNOLOGY AUSTRALIA 6671 SWINBURNE INSTITUTE OF TECH AUSTRALIA 7296 THE UNIVERSITY OF MELBOURNE AUSTRALIA 7317 UNIV OF MELBOURNE EXCHANGE PROGRAM AUSTRALIA 7287 UNIV OF NEW SO WALES EXCHG PROG AUSTRALIA 6737 UNIV OF QUEENSLAND EXCHANGE PROGRAM AUSTRALIA 6756 UNIV OF SYDNEY EXCHANGE PROGRAM AUSTRALIA 7289 UNIV OF WESTERN AUSTRALIA EXCHG PRO AUSTRALIA 7332 UNIVERSITY OF ADELAIDE AUSTRALIA 7142 UNIVERSITY OF CANBERRA AUSTRALIA 7027 UNIVERSITY OF NEW SOUTH WALES AUSTRALIA 7276 UNIVERSITY OF NEWCASTLE AUSTRALIA 6331 UNIVERSITY OF QUEENSLAND AUSTRALIA 7265 UNIVERSITY -

Erasmus in Valladolid
University of Valladolid Erasmus Programme WHERE IS VALLADOLID? Spain and Valladolid SPAIN WHERE IS VALLADOLID? WHERE IS VALLADOLID? WHERE IS VALLADOLID? Castile and Leon is one of the 17 Spanish regions The largest region in Spain 2.5 million inhabitants Nine provinces Avila, Burgos, Leon, Palencia (UVa), Salamanca, Segovia (UVa), Soria (UVa), Valladolid (UVa) and Zamora WHERE IS VALLADOLID? Capital of Castile and Leon Northwest of Madrid 350,000 inhabitants Splendorous past Purest Spanish spoken Large student population THE CITIES Valladolid, Palencia, Segovia and Soria LIFE IN VALLADOLID It was founded during the reign of Alfonso VI of Castille (11th century) Wedding of the Catholic Monarchs, death of Columbus, birth of Felipe II Culture, museums, archives, night life, music festivals Purest Spanish spoken Cervantes, Zorrilla, Delibes Known for its variety of wines and gastronomy Cold winters with temperatures below 0º to very hot summers with temperatures around 30º LIFE IN PALENCIA Inhabited since ancient times. Celtic tribes Vacceos. Romans, Visigoths, Arabs Rich in art and monuments First University of Spain 80,000 inhabitants Countryside: “Tierra de Campos”, Cristo del Otero, Road to Santiago, Roman Villages, Romanic Art Gastronomy LIFE IN SEGOVIA First historical reference to Segovia: 192 B.C. when the Celt Iberian inhabitants were defeated by Roman forces UNESCO World Heritage City Álcazar, cathedral, aqueduct Quiet provincial capital Gastronomy LIFE IN SORIA Small and peaceful city Amazing landscapes -

Experience Spanish in the 4 Universities of Castilla Y León
EXPERIENCE SPANISH IN THE 4 UNIVERSITIES EXPERIENCE SPANISH OF CASTILLA Y LEÓN IN THE 4 UNIVERSITIES OF CASTILLA Y LEÓN 4 cities, 4 experiences, one universal language 07 Registration and contacts Application form [scan this code to access the form]: CONTACTS: University of Burgos: [email protected] University of Valladolid: [email protected] University of León: [email protected] University of Salamanca: [email protected] 08 Programming The Spanish course taught the four public Universities of Castilla y EXPERIENCE SPANISH León will be held in an Avant guard classroom and will be complemen- IN THE 4 UNIVERSITIES ted by 6 theoretical-practical hours offering an approach to the history, art, language and literature of Castilla y León. OF CASTILLA Y LEÓN The package includes: Academic program and textbook, Scheduled activities, Transfer to and from the airport, Transfer to the 4 universities- Board and lodging costs. EXPERIENCE SPANISH IN THE 4 UNIVERSITIES OF CASTILLA Y LEÓN Group American students 02 Description of the course 04 Services Courses ELE A2 and B1 (80 hours + classes “in itinere” + cultural Four weeks Intensive Spanish, designed with the objective to provide Wifi on university campus. activities) the student with the necessary skills and knowledge to be able to Material for the course. Number of participants communicate within the level taught. Training of the four integrated Diploma granted by the 4 Universities. 15 students basic skills, strengthening of grammar and vocabulary through a Credits ECTS communicative context will be our major goals. Official reception. 12 The methodology follows a communicative and dynamic approach.. Transfer upon arrival at the Madrid-Barajas airport to the respective Scholarships From the beginning students will actively use the language, interacting offices of Burgos, Valladolid, León and Salamanca, and back to the Optional scholarships with their classmates and working in groups. -
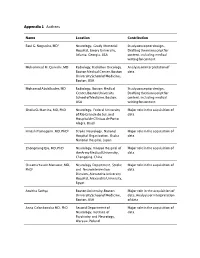
Appendix 1 Authors
Appendix 1 Authors Name Location Contribution a Raul G. Nogueira, MD Neurology, Grady Memorial Study concept or design, Hospital, Emory University, Drafting the manuscript for Atlanta, Georgia, USA content, including medical writing for content Muhammad M. Qureshi, MD Radiology, Radiation Oncology, Analysis or interpretation of Boston Medical Center, Boston data University School of Medicine, Boston, USA Mohamad Abdalkader, MD Radiology, Boston Medical Study concept or design, Center, Boston University Drafting the manuscript for School of Medicine, Boston, content, including medical USA writing for content Sheila O. Martins, MD, PhD Neurology, Federal University Major role in the acquisition of of Rio Grande do Sul, and data Hospital de Clínicas de Porto Alegre, Brazil b Hiroshi Yamagami, MD, PhD Stroke Neurology, National Major role in the acquisition of Hospital Organization, Osaka data National Hospital, Japan Zhongming Qiu, MD, PhD Neurology, Xinqiao Hospital of Major role in the acquisition of the Army Medical University, data Chongqing, China Ossama Yassin Mansour, MD, Neurology Department, Stroke Major role in the acquisition of c PhD and Neurointervention data Division, Alexandria University Hospital, Alexandria University, Egypt Anvitha Sathya Boston University, Boston Major role in the acquisition of University School of Medicine, data, Analysis or interpretation Boston, USA of data Anna Czlonkowska MD, PhD Second Department of Major role in the acquisition of Neurology, Institute of data Psychiatry and Neurology, Warsaw, Poland -

SPANISH COOPERATION on MATHEMATICS 2013 Next Activities Are Reported by Mercedes Siles and Begoña Vitoriano, Secretary and Pres
SPANISH COOPERATION ON MATHEMATICS 2013 Next activities are reported by Mercedes Siles and Begoña Vitoriano, secretary and president of the Commission for Development and Cooperation of the CE-MAT (Spanish Committee of Mathematics). We are sure that they are not all the Spanish cooperation developed in Mathematics with developing countries, but it can be seen a good approximation. As a general framework of cooperation, it should be remarked that Spain through the Ministerio of Economía y Competitividad is one of the supporters of CIMPA, and the different Spanish mathematical societies are member of CIMPA. LATINAMERICA 1) Spain-Central America One of the most ambitious processes in this region is a Central-American PhD programme on Mathematics. Putting together all their forces (PhDs in Panamá, El Salvador, Guatemala, Nicaragua, Honduras, Costa Rica and Dominican Republic) a common programme is being designed to be developed jointly. Several difficulties have to be solved because the different regulations of PhD studies in the different countries, but it looks that finally is going to be a reality, beginning in University of Panamá in 2014, with students and professors from different countries. It is expected that in 2015 it will begin also in El Salvador. Because the lack of enough PhDs in a country, they have designed a programme in which the students will take some courses in their original countries or other ones, but the thesis will be supervised by any of the professors included in the joint programme, depending on the speciality. Spanish professors are involved assisting to the design of the programme in different countries (Mercedes Siles in Panamá, Begoña Vitoriano, Javier Gallego and Juan Tejada in El Salvador…), adapted to the needs of each country. -

25 Years of Linking the Camino De Santiago and Higher Education
25 YEARS OF LINKING THE CAMINO DE SANTIAGO AND HIGHER EDUCATION COMPOSTELA GROUP OF UNIVERSITIES • 25 YEARS LINKING THE CAMINO DE SANTIAGO AND HIGHER EDUCATION compostelaGROUP OF UNIVERSITIES GRUPO DE UNIVERSIDADES A GRAPHIC HISTORY OF THE CGU TABLE OF CONTENTS EXECUTIVE COMMITTEES OF THE COMPOSTELA GROUP OF UNIVERSITIES . 5 PRESIDENTS OF THE CGU . 6 EXECUTIVE SECRETARIES OF THE CGU . 8 STEERING COMMITTEE OF THE CGU . 12 EXECUTIVE COMMITTEE OF THE CGU . 14 MILESTONES IN THE 25-YEAR HISTORY OF THE COMPOSTELA GROUP OF UNIVERSITIES . 23 1994 . 24 1996 . 26 2000 . 42 2001 . 46 2003 . 48 2004 . .52 . 2007 . 62 2010 . 66 2011 . 72 2014 . 76 2017 . 80 2018 . 88 2019 . 92 2 3 Executive OF THE COMPOSTELA GROUP OF UNIVERSITIES Committees 4 5 PRESIDENTS 1994 - 1999 OF THE CGU Marc Richelle UNIVERSITY OF LIÈGE 1999 - 2007 Michael Cooper KARLSTAD UNIVERSITY 2007 - 2015 Maurits van Rooijen UNIVERSITY OF WESTMINSTER (1993-2009) NYENRODE UNIVERSITY (2009-2012) GLOBAL UNIVERSITY SYSTEMS (2012 TO PRESENT) 2015 - PRESENT Marek Kręglewski ADAM MICKIEWICZ UNIVERSITY, POZNAN 6 7 EXECUTIVE 1995 - 1998 SECRETARIES OF THE CGU Álvaro de Mingo Díaz 1998 Manuel Freire-Garabal UNIVERSITY OF SANTIAGO DE COMPOSTELA 1999 - 2003 Marta González Vázquez 2004 - 2006 Enrique López Veloso UNIVERSITY OF SANTIAGO DE COMPOSTELA 2006 - 2010 Beatriz Iglesias Seoane 8 9 2012 - 2017 Isabel Lirola Delgado UNIVERSITY OF SANTIAGO DE COMPOSTELA 2017 - PRESENT María Teresa Carballeira Rivera UNIVERSITY OF SANTIAGO DE COMPOSTELA 10 11 STEERING 1993 - 1994 COMMITTEE Félix García Lausín María da Conceição Matos OF THE CGU UNIVERSITY JAUME I UNIVERSITY OF MINHO José Vicente Oliver Villanueva María José Crespo Allue UNIVERSITY GEORGIA-AUGUSTA GÖTTINGEN UNIVERSITY OF VALLADOLID Manuel Freire-Garabal Pierre Yvard UNIVERSITY OF SANTIAGO DE COMPOSTELA UNIVERSITY OF NANTES Marc Richelle Ramón Villares Paz UNIVERSITY OF LIÈGE UNIVERSITY OF SANTIAGO DE COMPOSTELA 12 13 EXECUTIVE 1994 - 1999 COMMITTEE Darío Villanueva Prieto Manuel Freire-Garabal OF THE CGU PERMANENT MEMBER . -

White-Rot Fungi Control on Populus Spp. Wood by Pressure Treatments with Silver Nanoparticles, Chitosan Oligomers and Propolis
Article White-Rot Fungi Control on Populus spp. Wood by Pressure Treatments with Silver Nanoparticles, Chitosan Oligomers and Propolis María Milagrosa Casado-Sanz 1, Iosody Silva-Castro 2, Laura Ponce-Herrero 3, Pablo Martín-Ramos 4,* , Jesús Martín-Gil 2 and Luis Acuña-Rello 1 1 Laboratorio de Tecnología de la Madera, Departamento de Ingeniería Agrícola y Forestal, Universidad de Valladolid, Avenida de Madrid 44, 34004 Palencia, Spain; [email protected] (M.-M.C.-S.); [email protected] (L.A.-R.) 2 Laboratorio de Tecnología Ambiental, Departamento de Ingeniería Agrícola y Forestal, Universidad de Valladolid, Avenida de Madrid 44, 34004 Palencia, Spain; [email protected] (I.S.-C.); [email protected] (J.M.-G.) 3 Sustainable Forest Management Research Institute, University of Valladolid—INIA, Avenida de Madrid 57, 34004 Palencia, Spain; [email protected] 4 EPS, Instituto Universitario de Investigación en Ciencias Ambientales de Aragón (IUCA), Universidad de Zaragoza, Carretera de Cuarte s/n, 22071 Huesca, Spain * Correspondence: [email protected]; Tel.: +34-974292668 Received: 10 September 2019; Accepted: 1 October 2019; Published: 7 October 2019 Abstract: There is growing interest in the development of non-toxic, natural wood preservation agents to replace conventional chemicals. In this paper, the antifungal activities of silver nanoparticles, chitosan oligomers, and propolis ethanolic extract were evaluated against white-rot fungus Trametes versicolor (L.) Lloyd, with a view to protecting Populus spp. wood. In order to create a more realistic in-service type environment, the biocidal products were assessed according to EN:113 European standard, instead of using routine in vitro antimicrobial susceptibility testing methods. -

Tomás Albaladejo Is Full Professor in Theory of Literature and Comparative Literature of the Autonomous University of Madrid. H
Tomás Albaladejo is Full Professor in Theory of Literature and Comparative Literature of the Autonomous University of Madrid. He has formerly taught at the University of Málaga, the University of Murcia, the University of Alicante and the University of Valladolid. He has held Visiting Positions at the University François Rabelais of Tours, at the University of Nottingham and at the University of Oxford. His current teaching at the Autonomous University of Madrid is in the field of Theory of Literature, Comparative Literature and Rhetoric. His subjects in the academic year 2020-2021, as well as in the last five academic years, are “Comparative literature: international spaces and literary interculturality” in the Degree in International Studies, “Foundations of literary analysis” in the Degree in Hispanic Studies, and “Rhetoric and argumentation” in the Degree in Modern Languages, Culture and Communication. His research lines are Theory of literature, Comparative literature, Theory of literary language, Linguistic and formal analysis of literature, Rhetoric, Literary translation theory, Narrative, Fictionality, Spanish contemporary poetry, Political discourse, Literature in international spaces, and Literature and discourse of conflict and post-conflict. He has been the Principal Researcher of several research projects. He is currently the Principal Research 1 of the research project “Analogy, equivalence, polyvalence and transferability as cultural-rhetoric and interdiscursive foundations of the art of language: literature, rhetoric and discourse” (Acronym: TRANSLATIO), whose Principal Research 2 is Francisco Javier Rodríguez Pequeño (Full Professor in Theory of Literature and Comparative Literature of the Autonomous University of Madrid. This project is a development and enlargement of the main results of the former research project “Metaphor as a component of Cultural Rhetoric. -

Academic Training in Spanish Universities for the Didactic Use of Cinema in Pre-School and Primary Education
Journal of Technology and Science Education JOTSE, 2021 – 11(1): 210-226 – Online ISSN: 2013-6374 – Print ISSN: 2014-5349 https://doi.org/10.3926/jotse.1162 ACADEMIC TRAINING IN SPANISH UNIVERSITIES FOR THE DIDACTIC USE OF CINEMA IN PRE-SCHOOL AND PRIMARY EDUCATION Alejandro Lorenzo-Lledó , Asunción Lledó , Gonzalo Lorenzo , Elena Pérez-Vázquez University of Alicante (Spain) [email protected], [email protected], [email protected], [email protected] Received November 2020 Accepted December 2020 Abstract One of the characteristic features of current society is the relevance of technology and audiovisual media. This fact has generated demands in the educational field to adapt objectives and methodologies to non-textual languages. Among the predominant audiovisual media is the cinema, which has many potentialities as a didactic resource. Therefore, the aim of this study was to find out the training that students of the Teacher’s Degree in Spanish universities receive for the didactic use of cinema through a nationwide research with survey design in which 4659 students, belonging to all the Autonomous Communities and 58 universities, participated. The questionnaire called Perceptions about the potentialities of cinema as a didactic resource in pre-school and primary classrooms (PECID) was designed ad hoc. This questionnaire, which consists of 45 items, has a section that deals with the training received for the educational use of cinema. The Spanish universities offering the Teacher Degree were identified and contacted for the dissemination of the questionnaire. The results obtained showed that 88.4% had not received training. Furthermore, 250 subjects were identified in which film content is taught, mostly in the second and third year and in the area of Didactics and School Organization. -

Scholars and Literati at the University of Valladolid (1280–1800)
Repertorium Eruditorum Totius Europae - RETE (2021) 1:11–18 11 https://doi.org/10.14428/rete.v1i0/Valladolid Licence CC BY-SA 4.0 Scholars and Literati at the University of Valladolid (1280–1800) David de la Croix Soraya Karioun IRES/LIDAM, UCLouvain In this note, we provide a summary description of the set of scholars and literati who taught at the University of Valladolid since its inception in the 13th century to the eve of the Industrial Revolution (1800). 1 The University Founded during the 13th century, the University of Valladolid is one of the oldest and most important universities in Spain. The university provided a home for professors and students, at a time when higher education was scattered all over the country, and provided a secular education, in a place where abbeys and episcopal establishments were predominant. With the support of the king and, later, the pope, the university built up a solid reputation intellectually, but also economically and culturally, while it closely participated in the development of the city (Fernández and González 1986). Unlike most old and prestigious universities elsewhere in Europe, the university of Valladolid was not implacably hostile towards the Jesuits, and permitted some Jesuits to ll university positions (Grendler 2019). 2 Sources The three-volume book « Historia de la Universidad de Valladolid » was written by Mariano Alcocer Martínez, a Spanish historian, archivist, and bibliographer. The third volume deals exclusively with the professors of the university. The author provides a complete historical picture through vacan- cies and appointments between 1404 and 1832, followed by a methodological presentation of the professors’ biographies (Alcocer Martinéz 1918).