Abiogenesis and Photostimulated Heterogeneous Reactions in The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Donor-Acceptor Interaction and Photochemistry of Polymethylene-Linked Bichromophores in Solution
9042 J. Am. Chem. Soc. 1996, 118, 9042-9051 Donor-Acceptor Interaction and Photochemistry of Polymethylene-Linked Bichromophores in Solution Song-lei Zhang, Matthew J. Lang, Steven Goodman,† Christopher Durnell, Vlastimil Fidlar,‡ Graham R. Fleming, and Nien-chu C. Yang* Contribution from the Department of Chemistry, UniVersity of Chicago, Chicago, Illinois 60637 ReceiVed July 17, 1996X Abstract: The ground-state and the excited-state spectroscopic properties of four series of polymethylene-linked anthracene-dialkylaniline bichromophores were compared as a probe to the relationship between energetics and distance in photoinduced electron transfer (PET). The results demonstrate that, when the energy level of the charge- transfer (CT) state is lowered below that of the localized excited state by appropriate substituents, there is a strong electron-donor-acceptor (EDA) interaction in the ground state which is absent in other bichromophores. Absorption and fluorescence excitation studies revealed that there is an unusually strong EDA interaction in the ground state of A-2 which is absent in other members in the A series. When A-2 is excited directly into this EDA absorption, it exhibits two CT emissions, one at 490 nm and the other at 605 nm. The quantum yield (τf) and the lifetime (Φf) of the two emissions are dependent on the viscosity of the alkane solvent. The Φf and the τf of the 490 nm emission increased when the solvent viscosity was increased; however, those of the 605 nm emission remained essentially unchanged. The risetime of the 605 nm emission is 420 ps, but that of the 490 nm emission is instrument-function limiting. -

Opportunities for Catalysis in the 21St Century
Opportunities for Catalysis in The 21st Century A Report from the Basic Energy Sciences Advisory Committee BASIC ENERGY SCIENCES ADVISORY COMMITTEE SUBPANEL WORKSHOP REPORT Opportunities for Catalysis in the 21st Century May 14-16, 2002 Workshop Chair Professor J. M. White University of Texas Writing Group Chair Professor John Bercaw California Institute of Technology This page is intentionally left blank. Contents Executive Summary........................................................................................... v A Grand Challenge....................................................................................................... v The Present Opportunity .............................................................................................. v The Importance of Catalysis Science to DOE.............................................................. vi A Recommendation for Increased Federal Investment in Catalysis Research............. vi I. Introduction................................................................................................ 1 A. Background, Structure, and Organization of the Workshop .................................. 1 B. Recent Advances in Experimental and Theoretical Methods ................................ 1 C. The Grand Challenge ............................................................................................. 2 D. Enabling Approaches for Progress in Catalysis ..................................................... 3 E. Consensus Observations and Recommendations.................................................. -
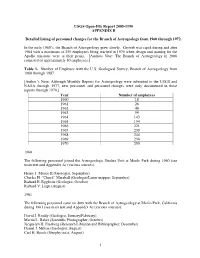
USGS Open-File Report 2005-1190, Appendix A
USGS Open-File Report 2005-1190 APPENDIX B Detailed listing of personnel changes for the Branch of Astrogeology from 1960 through 1972. In the early 1960’s, the Branch of Astrogeology grew slowly. Growth was rapid during and after 1964 with a maximum of 250 employees being reached in 1970 when design and training for the Apollo missions were at their peaks. [Authors Note: The Branch of Astrogeology in 2006 consisted of approximately 80 employees.] Table 1. Number of Employee with the U.S. Geological Survey, Branch of Astrogeology from 1960 through 1987 [Author’s Note: Although Monthly Reports for Astrogeology were submitted to the USGS and NASA through 1977, new personnel and personnel changes were only documented in these reports through 1970.] Year Number of employees 1960 18 1961 26 1962 40 1963 59 1964 143 1965 154 1966 221 1967 239 1968 244 1969 234 1970 250 1960 The following personnel joined the Astrogeologic Studies Unit at Menlo Park during 1960 (see main text and Appendix A) (various sources): Henry J. Moore II (Geologist, September) Charles H. “Chuck” Marshall (Geologist/Lunar mapper; September) Richard E. Eggleton (Geologist, October) Richard V. Lugn (August) 1961 The following personnel came on duty with the Branch of Astrogeology at Menlo Park, California during 1961 (see main text and Appendix A) (various sources): David J. Roddy (Geologist; January/February) Martin L. Baker (Scientific Photographer; October) Jacquelyn H. Freeberg (Research Librarian and Bibliographer; December) Daniel J. Milton (Geologist; August) Carl H. Roach (Geophysicist; August) 1 Maxine Burgess (Secretary) 1962 The following was taken from the Branch of Astrogeology Monthly Report for April 1962 from Chief, Branch of Astrogeology to V.E. -

Dehydrogenation by Heterogeneous Catalysts
Dehydrogenation by Heterogeneous Catalysts Daniel E. Resasco School of Chemical Engineering and Materials Science University of Oklahoma Encyclopedia of Catalysis January, 2000 1. INTRODUCTION Catalytic dehydrogenation of alkanes is an endothermic reaction, which occurs with an increase in the number of moles and can be represented by the expression Alkane ! Olefin + Hydrogen This reaction cannot be carried out thermally because it is highly unfavorable compared to the cracking of the hydrocarbon, since the C-C bond strength (about 246 kJ/mol) is much lower than that of the C-H bond (about 363 kJ/mol). However, in the presence of a suitable catalyst, dehydrogenation can be carried out with minimal C-C bond rupture. The strong C-H bond is a closed-shell σ orbital that can be activated by oxide or metal catalysts. Oxides can activate the C-H bond via hydrogen abstraction because they can form O-H bonds, which can have strengths comparable to that of the C- H bond. By contrast, metals cannot accomplish the hydrogen abstraction because the M- H bonds are much weaker than the C-H bond. However, the sum of the M-H and M-C bond strengths can exceed the C-H bond strength, making the process thermodynamically possible. In this case, the reaction is thought to proceed via a three centered transition state, which can be described as a metal atom inserting into the C-H bond. The C-H bond bridges across the metal atom until it breaks, followed by the formation of the corresponding M-H and M-C bonds.1 Therefore, dehydrogenation of alkanes can be carried out on oxides as well as on metal catalysts. -

Cometary Panspermia a Radical Theory of Life’S Cosmic Origin and Evolution …And Over 450 Articles, ~ 60 in Nature
35 books: Cosmic origins of life 1976-2020 Physical Sciences︱ Chandra Wickramasinghe Cometary panspermia A radical theory of life’s cosmic origin and evolution …And over 450 articles, ~ 60 in Nature he combined efforts of generations supporting panspermia continues to Prof Wickramasinghe argues that the seeds of all life (bacteria and viruses) Panspermia has been around may have arrived on Earth from space, and may indeed still be raining down some 100 years since the term of experts in multiple fields, accumulate (Wickramasinghe et al., 2018, to affect life on Earth today, a concept known as cometary panspermia. ‘primordial soup’, referring to Tincluding evolutionary biology, 2019; Steele et al., 2018). the primitive ocean of organic paleontology and geology, have painted material not-yet-assembled a fairly good, if far-from-complete, picture COMETARY PANSPERMIA – cultural conceptions of life dating back galactic wanderers are normal features have argued that these could not into living organisms, was first of how the first life on Earth progressed A SOLUTION? to the ideas of Aristotle, and that this of the cosmos. Comets are known to have been lofted from the Earth to a coined. The question of how from simple organisms to what we can The word ‘panspermia’ comes from the may be the source of some of the have significant water content as well height of 400km by any known process. life’s molecular building blocks see today. However, there is a crucial ancient Greek roots ‘sperma’ meaning more hostile resistance the idea of as organics, and their cores, kept warm Bacteria have also been found high in spontaneously assembled gap in mainstream understanding - seed, and ‘pan’, meaning all. -

Sustainable Catalysis
Green Process Synth 2016; 5: 231–232 Book review Sustainable catalysis DOI 10.1515/gps-2016-0016 Sustainable catalysis provides an excellent in-depth over- view of the applications of non-endangered elements in Michael North (Ed.) all aspects of catalysis from heterogeneous to homogene- Sustainable catalysis: with non-endangered metals ous, from catalyst supports to ligands. The Royal Society of Chemistry, 2015 Four volumes of the book series are divided into RSC Green Chemistry series nos. 38–41 two sub-topics devoted to (i) metal-based catalysts and Part 1 (ii) catalysts without metals or other endangered ele- Print ISBN: 978-1-78262-638-1 ments. Each sub-topic comprises of two books due to the PDF eISBN: 978-1-78262-211-6 detailed analysis of catalytic applications described. All Part 2 chapters are written by the world-leading researchers in Print ISBN: 978-1-78262-639-8 the area; however, covering far beyond the scope of their PDF eISBN: 978-1-78262-642-8 immediate research interests, which makes the book par- ticularly valuable for a wide range of readers. Sustainable catalysis: without metals or other Sustainable catalysis: with non-endangered metals endangered elements begins with a very brief chapter which describes the Part 1 concept of elemental sustainability, overviewing the Print ISBN: 978-1-78262-640-4 abundance of the critical elements and perspectives on PDF eISBN: 978-1-78262-209-3 sustainable catalysts. The chapters follow the groups of EPUB eISBN: 978-1-78262-752-4 the periodic table from left to right, from alkaline metals to Part 2 lead spanning 22 chapters. -

The Early History of Catalysis
The Early History of Catalysis By Professor A. J. B. Robertson Department of Chemistry, King’s College, London One hundred and forty years ago it was Berzelius proceeded to propose the exist- possible for one man to prepare an annual ence of a new force which he called the report on the progress of the whole of “catalytic force” and he called “catalysis” the chemistry, and for many years this task was decomposition of bodies by this force. This undertaken by the noted Swedish chemist is probably the first recognition of catalysis J. J. Berzelius for the Stockholm Academy of as a wide-ranging natural phenomenon. Sciences. In his report submitted in 1835 and Metallic catalysts had in fact been used in published in 1836 Berzelius reviewed a num- the laboratory before 1800 by Joseph Priestley, ber of earlier findings on chemical change in the discoverer of oxygen, and by the Dutch both homogeneous and heterogeneous sys- chemist Martinus van Marum, both of whom tems, and showed that these findings could be made observations on the dehydrogenation of rationally co-ordinated by the introduction alcohol on metal catalysts. However, it seems of the concept of catalysis. In a short paper likely that these investigators regarded the summarising his ideas on catalysis as a new metal merely as a source of heat. In 1813, force, he wrote (I): Louis Jacques Thenard discovered that ammonia is decomposed into nitrogen and “It is, then, proved that several simple or compound bodies, soluble and insoluble, have hydrogen when passed over various red-hot the property of exercising on other bodies an metals, and ten years later, with Pierre action very different from chemical affinity. -

Cosmochemistry Cosmic Background Radia�On
6/10/13 Cosmochemistry Cosmic background radiaon Dust Anja C. Andersen Niels Bohr Instute University of Copenhagen hp://www.dark-cosmology.dk/~anja Hauser & Dwek 2001 Molecule formation on dust grains Multiwavelenght MW 1 6/10/13 Gas-phase element depleons in the Concept of dust depleon interstellar medium The depleon of an element X in the ISM is defined in terms of (a logarithm of) its reducon factor below the expected abundance relave to that of hydrogen if all of the atoms were in the gas phase, [Xgas/H] = log{N(X)/N(H)} − log(X/H) which is based on the assumpon that solar abundances (X/H)are good reference values that truly reflect the underlying total abundances. In this formula, N(X) is the column density of element X and N(H) represents the column density of hydrogen in both atomic and molecular form, i.e., N(HI) + 2N(H2). The missing atoms of element X are presumed to be locked up in solids within dust grains or large molecules that are difficult to idenfy spectroscopically, with fraconal amounts (again relave to H) given by [Xgas/H] (Xdust/H) = (X/H)(1 − 10 ). Jenkins 2009 Jenkins 2009 2 6/10/13 Jenkins 2009 Jenkins 2009 The Galacc Exncon Curve Extinction curves measure the difference in emitted and observed light. Traditionally measured by comparing two stars of the same spectral type. Galactic Extinction - empirically determined: -1 -1 <A(λ)/A(V)> = a(λ ) + b(λ )/RV (Cardelli et al. 1999) • Bump at 2175 Å (4.6 µm-1) • RV : Ratio of total to selective extinction in the V band • Mean value is RV = 3.1 (blue) • Low value: RV = 1.8 (green) (Udalski 2003) • High value: RV = 5.6-5.8 (red) (Cardelli et al. -

Lifetimes of Interstellar Dust from Cosmic Ray Exposure Ages of Presolar Silicon Carbide
Lifetimes of interstellar dust from cosmic ray exposure ages of presolar silicon carbide Philipp R. Hecka,b,c,1, Jennika Greera,b,c, Levke Kööpa,b,c, Reto Trappitschd, Frank Gyngarde,f, Henner Busemanng, Colin Madeng, Janaína N. Ávilah, Andrew M. Davisa,b,c,i, and Rainer Wielerg aRobert A. Pritzker Center for Meteoritics and Polar Studies, The Field Museum of Natural History, Chicago, IL 60605; bChicago Center for Cosmochemistry, The University of Chicago, Chicago, IL 60637; cDepartment of the Geophysical Sciences, The University of Chicago, Chicago, IL 60637; dNuclear and Chemical Sciences Division, Lawrence Livermore National Laboratory, Livermore, CA 94550; ePhysics Department, Washington University, St. Louis, MO 63130; fCenter for NanoImaging, Harvard Medical School, Cambridge, MA 02139; gInstitute of Geochemistry and Petrology, ETH Zürich, 8092 Zürich, Switzerland; hResearch School of Earth Sciences, The Australian National University, Canberra, ACT 2601, Australia; and iEnrico Fermi Institute, The University of Chicago, Chicago, IL 60637 Edited by Mark H. Thiemens, University of California San Diego, La Jolla, CA, and approved December 17, 2019 (received for review March 15, 2019) We determined interstellar cosmic ray exposure ages of 40 large ago. These grains are identified as presolar by their large isotopic presolar silicon carbide grains extracted from the Murchison CM2 anomalies that exclude an origin in the Solar System (13, 14). meteorite. Our ages, based on cosmogenic Ne-21, range from 3.9 ± Presolar stardust grains are the oldest known solid samples 1.6 Ma to ∼3 ± 2 Ga before the start of the Solar System ∼4.6 Ga available for study in the laboratory, represent the small fraction ago. -

Physical Organic Chemistry
CHM 8304 Physical Organic Chemistry Catalysis Outline: General principles of catalysis • see section 9.1 of A&D – principles of catalysis – differential bonding 2 Catalysis 1 CHM 8304 General principles • a catalyst accelerates a reaction without being consumed • the rate of catalysis is given by the turnover number • a reaction may alternatively be “promoted” (accelerated, rather than catalysed) by an additive that is consumed • a heterogeneous catalyst is not dissolved in solution; catalysis typically takes place on its surface • a homogeneous catalyst is dissolved in solution, where catalysis takes place • all catalysis is due to a decrease in the activation barrier, ΔG‡ 3 Catalysts • efficient at low concentrations -5 -4 -5 – e.g. [Enz]cell << 10 M; [Substrates]cell < 10 - 10 M • not consumed during the reaction – e.g. each enzyme molecule can catalyse the transformation of 20 - 36 x 106 molecules of substrate per minute • do not affect the equilibrium of reversible chemical reactions – only accelerate the rate of approach to equilibrium end point • most chemical catalysts operate in extreme reaction conditions while enzymes generally operate under mild conditions (10° - 50 °C, neutral pH) • enzymes are specific to a reaction and to substrates; chemical catalysts are far less selective 4 Catalysis 2 CHM 8304 Catalysis and free energy • catalysis accelerates a reaction by stabilising a TS relative to the ground state ‡ – free energy of activation, ΔG , decreases – rate constant, k, increases • catalysis does not affect the end point -

C-2 Ch 2 & 3 Atoms & Molecules
Molecular and Supramolecular Photochemistry Modern Molecular Photochemistry of Organic Molecules Or Principles of Photochemistry N. J. Turro, V. Ramamurthy and J. C. Scaiano Chapters 1 & 2 Photochemistry Interaction of Light with Matter (Molecules) • Organic Photochemistry • Inorganic Photochemistry • Photobiology What is the difference between thermochemistry and photochemistry? • Mode of activation • Activated by collisions (thermo) • Activated by light (photo) • Selectivity in activation • Entire molecule gets activated • Only the chromophore that absorbs the light gets activated • Energy distribution • Energy used for vibrational/rotational transition • Energy used for electronic transition only Visualization of Thermal Reactions • Transition state connects a single reactant to a single product (intermediate) and it is a saddle point along the reaction course. • Collisions are a reservoir of continuous energy (~ 0.6 kcal/mol per impact). • Collisions can add or remove energy from a system. • Concerned with a single surface. Visualization of Photochemical Reactions We need to deal with two surfaces (ground and excited state. Adiabatic Diabatic Photochemistry starts with interaction of a Photon with a Molecule • What is a photon? • What is a molecule? • How do they interact? • What are the consequences of interaction? The Basic Laws of Photochemistry Grotthuss-Draper law The First Law of Photochemistry: light must be absorbed for photochemistry to occur. Grotthus Drapper Stark-Einstein law The Second Law of Photochemistry: for each photon of light absorbed by a chemical system, only one molecule is activated for a photochemical Stark Einstein reaction. The Light Paradigm (500 BC-1850 AD) 500 2018 BC AD 55 BC 1000 1500 1700 Lucretius Newton (1643-1727) Maxwell (1831-1879) Particles! Waves! …but then came the 1900s - new people, tools, and paradigms! Paradigm 1800s: Light consists of waves (energy propagated by waves): Energy is spread over space like a liquid. -

Interstellar Dust Within the Life Cycle of the Interstellar Medium K
EPJ Web of Conferences 18, 03001 (2011) DOI: 10.1051/epjconf/20111803001 C Owned by the authors, published by EDP Sciences, 2011 Interstellar dust within the life cycle of the interstellar medium K. Demyk1,2,a 1Université de Toulouse, UPS-OMP, IRAP, Toulouse, France 2CNRS, IRAP, 9 Av. colonel Roche, BP. 44346, 31028 Toulouse Cedex 4, France Abstract. Cosmic dust is omnipresent in the Universe. Its presence influences the evolution of the astronomical objects which in turn modify its physical and chemical properties. The nature of cosmic dust, its intimate coupling with its environment, constitute a rich field of research based on observations, modelling and experimental work. This review presents the observations of the different components of interstellar dust and discusses their evolution during the life cycle of the interstellar medium. 1. INTRODUCTION Interstellar dust grains are found everywhere in the Universe: in the Solar System, around stars at all evolutionary stages, in interstellar clouds of all kind, in galaxies and in the intergalactic medium. Cosmic dust is intimately mixed with the gas-phase and represents about 1% of the gas (in mass) in our Galaxy. The interstellar extinction and the emission of diffuse interstellar clouds is reproduced by three dust components: a population of large grains, the BGs (Big Grains, ∼10–500 nm) made of silicate and a refractory mantle, a population of carbonaceous nanograins, the VSGs (Very Small Grains, 1–10 nm) and a population of macro-molecules the PAHs (Polycyclic Aromatic Hydrocarbons) [1]. These three components are more or less abundant in the diverse astrophysical environments reflecting the coupling of dust with the environment and its evolution according to the physical and dynamical conditions.