Engineering Handbook for the Designer of Hydro-Pneumatic Applications in Engineered Mechanical Systems…
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Experimental and Computational Study of Indirect Expansion Solar Assisted Heat Pump System with Latent Heat Storage for Domestic Hot Water Production
Experimental and computational study of indirect expansion solar assisted heat pump system with latent heat storage for domestic hot water production A thesis submitted for the degree of Doctor of Philosophy (PhD) by Walid Mohamed Khalil Abdalla Youssef College of Engineering, Design, and Physical Sciences Brunel University London Abstract Solar assisted heat pump (SAHP) systems have been widely applied in domestic hot water (DHW) production due to their sustainability and stability in operations. However, their performance efficiency requires further improvement using advanced technologies such as energy storage with phase change materials (PCM) and optimal system controls. Undoubtedly, employing PCMs for latent heat storage (LHS) application has a great potential to improve a solar thermal application performance. Despite this fact, the use of PCM in this area is quite limited due to the poor thermal conductivity of available PCMs. Therefore, heat transfer enhancement is one of the essential strategies that can overcome this obstacle. Accordingly, a test rig of a new indirect expansion solar assisted heat pump (IDX-SAHP) system has been designed, built and instrumented. The system can handle heating capacity up to 9 kW. The IDX-SAHP system consists of three operational loops: solar thermal, solar-air assisted heat pump and load profile. A 2 kW PCM heat exchanger (HX) was purposely designed and installed in the system solar thermal loop to store solar energy, when applicable, and release heat when required by the heat pump. The PCM HX is employed with a novel heat transfer enhancement method. The maximum coefficient of performance (COP) of the IDX-SHAP system reached 4.99 during the sunny day with the PCM (HX) integration. -

Taeevo Tech Is an Air Cooled Liquid Chiller, Designed for Industrial Use and for Installation in an External Environment
PROCESS COOLING SOLUTIONS TAEevo Air cooled industrial chillers with Scroll compressors - R410A Nominal cooling capacity 1.7 – 53.6 Tons Cooling your industry, optimising your process. TAEevo Tech is an air cooled liquid chiller, designed for industrial use and for installation in an external environment. A broad range of options available in product configuration and accessories in kit form, complete the already generous standard equipment and allow this machine to meet the majority of requirements of industrial applications. TAEevo Tech is therefore the solution for all applications that require high performance, reliability, continuity of operation and reduced management costs. Higher energy efficiency Respect for the Environment IC208CX microprocessor control User friendly Thanks especially to the ener- The eco-friendly refrigerant R410A TAEevo Tech features a new ad- The operation principle of the unit gy efficient scroll compressors, (ODP=0) with outstanding heat vanced microprocessor control is displayed in a simple and intui- the oversized evaporator and the conductivity, coupled with the low technology, with all models fitted tive synoptic sticker with new de- refrigerant R410A, TAEevo Tech absorbed power level of the scroll with a unique IC208CX digital con- sign. The meaning of the codes of achieves leading energy efficiency compressors, reduce the environ- trol. A comprehensive digital dis- the main alarms shown on the dis- levels. This is mated to low main- mental impact, minimizing the en- play keeps the user fully informed play of the controller are therefore tenance needs, ensuring TAEevo ergy waste. Recyclable and high concerning the correct operation easy to understand, even without Tech is a highly economical long- quality materials ensure respect for of the unit, warnings and alarms. -

Preparation and Startup of New Systems This Document Is Offered As a General Guideline to Starting a New Or Freshly Filled System
New System Startup Preparation and Startup of New Systems This document is offered as a general guideline to starting a new or freshly filled system. Please follow proper safety procedures and consult your system manufacturer for specifics on your system and what steps need to be taken to ensure a smooth and safe start up. System Preparation 1. A general inspection of the system before proceeding is recommended. Ensure all pipes, flanges, valves etc. are properly installed/tightened and valves are in their designated open/closed position for charging the system with fluid. 2. Inspect system for any water that may have been introduced during construction, pressure testing etc. Check with your system manufacturer for specific procedures but opening low point drains and blowing nitrogen or dry air (moist/standard air could introduce more water) through the system is generally recommended to ensure the system is dry. Filling the System Once the system’s integrity has been checked and all water removed it’s time to start filling the system. 1. Consider the ambient/fluid temperature and its affect on the fluid’s viscosity to ensure adequate pumps are available to start charging the system with fluid. Consult your specific Duratherm fluid property chart for its viscosity at your specific fill temperature. 2. Open all high point vents and valves to the various system ‘users’. 3. Filling the system should be done through the lowest point in the system in order to prevent air pockets - this is generally at the pump level and in some cases the system pump may be appropriate for filling the system. -

Best Practices Manual for Solar Hot Water Systems
Best Practices Manual for Solar Hot Water Systems Updated: 10-25-2011 Introduction: This manual was developed as a tool to assist solar thermal designers and installers as a guideline to provide the most reliable solar hot-water systems possible. The material presented here is not intended to be used as a list of system requirements or as a type of solar code. Rather, it was assembled with the input of many parties to share lessons learned in the field. It is not inclusive and is a work in progress. This manual was developed in Wisconsin where some parts of the state have over 10,000 heating degree days and where winter temperatures regularly fall below -30°F. In fact, the record coldest temperature recorded in Wisconsin was - 55°F. During the summer, temperatures can rise above 100°F. While most climates are not this severe, the practices outlined in this manual will be helpful for system designs in all cold climates as well as in warm climates. A properly designed solar hot-water system must not only function properly during extreme cold and hot environmental circumstances, it must also be able to safely endure sustained periods of low or no hot water draw without damage or overheating. A best practice is defined as: • A practice that is most appropriate under the circumstances. • A technique or methodology that, through experience and research, has reliably led to a desired or optimum result. Overview: A well-designed solar water heating system that is appropriate for the climate where it is located and is properly installed with appropriate solar rated components will last for many years. -

Thermal Expansion Products
Now includes our LEAD FREE * product offering Thermal Expansion Products watts.com Table of Contents General Information . 2 – 4 Potable Hot Water System . 5 Series PLT Potable water expansion tanks . 6 Series DETA ASME pressurized potable water expansion tanks . 7 Series LFBRV Combination ball valve and relief valve . 8 Series Governor 80M2 Ball cock and thermal expansion relief valve . 9 Series LF530C Calibrated pressure relief valve . 10 Nonpotable Hot Water System . 11 Series 276H300/IWTG Water pressure test gauges . 12 Series SCV Service check valves . 13 Series ETX-ASF Combination packages . 14 Series HPX Boiler trim-out packages . 14 Boiler Header Modules Pro Hydronic packages . 15 Series ETX, ETSX pressurized expansion tanks for heating and cooling systems . 16 Series ETA ASME pressurized nonpotable water expansion tanks . 18 Series ET-RA ASME pressurized nonpotable water expansion tanks . 19 Watts product specifications in U.S. customary units and metric are approximate and are provided for reference only. Watts reserves the right to change or modify product design, construction, specifications, or materials without prior notice and without incurring any obligation to make such changes and modifications on Watts products previously or subsequently sold. 1 General Information General Information What is Thermal Expansion? When water is heated, it expands . For example, water heated from 90ºF (32ºC) to a thermostat setting of 140ºF (60ºC) in a 40 gallon hot water heater will expand by almost one-half gallon . This is because when water is heated, its density decreases and its volume expands (see fig . 1) . Since water is not compressible, the extra volume created by expansion must go someplace . -

AHE-375-D03 Installation Manual LTE-907-722 Rev B
INSTALLATION MANUAL © 2011 Aqua-Hot Heating Systems Inc. CAUTION: The Aqua-Hot tank and heating loop operate at 0.0 psi (zero pressure system). Air pressure applied to the tank MUST NOT exceed 20 psi. Excess pressure will result in internal damage. CAUTION: Before welding or plasma cutting on any coach; it is necessary to disconnect the electric wiring to the Aqua-Hot System. Failure to disconnect the wires from the Aqua-Hot System before using a welder or a plasma cutter on the coach may cause damage to the Aqua-Hot. TABLE OF CONTENTS Aqua-Hot 375-D Installation Manual Introduction ........................................................................................................................................... 5 Section 1: Aqua-Hot Hydronic Heating System Technical Information .......................................................................................................................... 6 Section 2: Aqua-Hot Installation Installing the Mounting Tray and the Aqua-Hot .............................................................................. 10 Overall Aqua-Hot Dimensions ........................................................................................................... 12 Installing the Cable-Clamp Fittings .................................................................................................... 14 Installing the Wires into the Terminal Blocks .................................................................................. 14 Installing the Expansion Tank ........................................................................................................... -

Commercial Hot Water Heat Exchanger Installation Manual
COMMERCIAL HOT WATER HEAT EXCHANGER INSTALLATION MANUAL COMMERCIAL HOT WATER HEAT EXCHANGER INSTALLATION MANUAL INDEX DESCRIPTION PAGE RATINGS AND DIMENSIONS ……………………………………………………………….……………………………………………………………………………………………………… 1 INSTALLATION & RECOMMENDED PIPING INSTALLATION WITH HOT WATER BOILERS ………………………………………………………………………………………………………………………………………………… 2 RECOMMENDED NEAR UNIT PIPING: ‐ ONE H2OMAX UNIT ……………………………………………………………………………………..………………………………………………………………… 3 ‐ MULTIPLE H2OMAX UNITS ……………………………………………………………………...……………………………………………………………………… 4 RECOMMENDED PIPING WITH HOT WATER BOILERS: ‐ (1) BOILER AND (1) HEAT EXCHANGER ‐ HYDRONIC HEATING AND DOMESTIC HOT WATER (DHW) ……………………………… 5 ‐ MULTIPLE BOILERS AND MULTIPLE HEAT EXCHANGERS ‐ HYDRONIC HEATING AND DHW ……………………………………………… 6 ALTERNATIVE PIPING SCHEMATICS WITH HOT WATER BOILERS: ‐ (1) BOILER AND (1) HEAT EXCHANGER ‐ HYDRONIC HEATING, DHW AND BUFFERING …………………………………………………… 7 ‐ (1) BOILER, (1) HEAT EXCHANGER & (1) STORAGE TANK ‐ HYDRONIC HEATING, DHW AND BUFFERING ………………………… 8 ‐ (1) BOILER AND (1) HEAT EXCHANGER ‐ HYDRONIC HEATING AND DHW ………………………………………………………………………… 9 ‐ (1) BOILER AND (2) HEAT EXCHANGERS ‐ HYDRONIC HEATING AND DHW ……………………………………………………………………… 9 INSTALLING ANTI‐SCALDING VALVE ………………………………………………………………………………….………………………………………………………………………… 10 INSTALLATION WITH LOW PRESSURE STEAM BOILERS ………………………………………………………………………………………………………………………………… 11 RECOMMENDED PIPING SCHEMATIC WITH LOW PRESSURE STEAM BOILERS: ‐ (1) BOILER AND (1) HEAT EXCHANGER ‐ DHW ……………………………………………………………………...………………………………………… -

Installation Instructions and Use & Care Guide
A.O. Smith – Voltex™ Hybrid Electric Heat Pump Water Heater Installation Instructions and Use & Care Guide To obtain technical, warranty or service assistance during or after the installation of this water heater, call toll free 1-800-527-1953 When calling for assistance, please have the following information ready: 1. Model number 2. 7 Digit product number 3. Serial number 4. Date of installation 5. Place of Purchase Table of Contents Page Water Heater Safety ............................................................................... 2 Installing Your Water Heater .................................................................3-9 Consumer Information ................................................................ 3 Consumer Responsibilities ......................................................... 3 Unpacking Instructions ............................................................3-4 Location Requirements ............................................................... 4 Confined Space Installation.......................................................5-6 Water System Piping ................................................................7-8 Temperature & Pressure Relief Valve .....................................9-10 Electrical Requirements ....................................................... 10-11 Installation Checklist .............................................................................. 12 Operating Your Water Heater ...........................................................13-15 Before Using ............................................................................ -
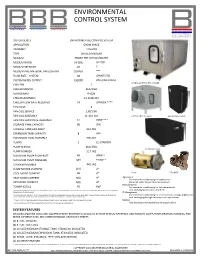
1316-002 Data Sheet
ENVIRONMENTAL CONTROL SYSTEM ROI air ionization SSI BEQUIPPED BEQUIPPED BEQUIPPED BROTHER BINDUSTRIAL EQUIPMENT SYSTEM SERIES ENVIRONMENTAL CONTROL SYSTEM APPLICATION GROW SPACE ASSEMBLY 1316-002 TYPE CHILLED MEDIUM MEDIUM PROPYLENE GLYCOL/WATER MEDIUM RATIO 33.00% GLYCOL MEDIUM SETPOINT 46 F MEDIUM PIPE MATERIAL APPLICATION COPPER *** FLOW RATE - SYSTEM 48 GPM/TOTAL SYSTEM RATED OUTPUT 192000 BTU (FAN COILS) MODULAR PROCESS CHILLER CHILLERS 2 CHILLER SERVICE 460/3/60 REFRIGERANT R422B CHILLER ASSEMBLY 11-1138-313 CHILLER FLOW RATE REQUIRED 24 GPM/EA.**** FAN COILS 4 FAN COIL SERVICE 230/1/60 FAN COIL ASSEMBLY 11-334-103 CLEAN AIR FAN COIL EXPANSION TANK FAN COIL FLOW RATE REQUIRED 12 GPM**** STORAGE TANK CAPACITY 88 GAL STORAGE TANK ASSEMBLY 910-001 EXPANSION TANK CAPACITY 8 GAL EXPANSION TANK ASSEMBLY 905-001 PUMPS 2 (1) STANDBY PUMP SERVICE 460/3/60 STORAGE TANK PUMP NUMBER 517-002 MAXIMUM PUMP FLOW RATE 48 GPM** MAXIMUM PUMP PRESSURE 184 FT HD** STRAINER NUMBER 445-001 PUMP MOTOR CURRENT 10.0 A* COOL MODE CURRENT 68 A* PUMP STRAINER HEAT MODE CURRENT N/A A* Agriculture Environmental conditioning for hydroponics DRY MODE CURRENT N/A A* Grow Pod water temperature maintenance Manufacturing POWER (COOL) 40 kW* Environmental conditioning for finished products *CURRENT/POWER ARE STATED FOR SYSTEM EQUIPMENT ONLY AT FULL SYSTEM OPERATION. VALUES ARE CALCULATED AT DESIGN POINT SPECIFICATIONS, SEE EQUIPMENT Heat exchanging for process machines NAMEPLATES FOR SERVICE SIZING IT Management **PUMP IS SPECIFIED FOR 200 FT. OF 2" CHILLED MEDIUM PIPE -

Closed Cooling System Overview
Why Does My BMW Expansion Tank Blow Up? Introduction For a decade and a half it’s been a real issue with the e46 chassis engines but other chassis have seen their fair share of this phenomenon. Lately, around BMW forums (one in particular) the issue of sourcing why this happens has gotten quite hysterical with folks rigging up pressure gauges and LED’s to monitor the internal pressures of the cooling system. Users are quoting all types of scientific formulas and hypothesis trying to find common criteria and making defacto interpretations from individual experiments. It really is quite funny. But why do BMW Expansion Tanks (ET’s for the BMW enthusiast) blow up? The reason is actually very simple – they are overfilled and have too much coolant in the cooling system with no room to expand. Yes it’s that simple. I’ll bet most reading this paper think they fully understand the BMW cooling system in their car but do you? So you know it’s a “closed cooling system”? However do you know what that actually infers….”closed”? I’d hazard that many do not, especially given the flurry of users replacing their stock ET caps with lower pressure rating release ET caps. Many who replaced their caps believe that pressure will be released (to atmosphere) like an old Open cooling system should pressures build up in excess within the cooling system. It will not. The PSI rating (2 Bar, 1.6 bar, 1.2 bar etc.) is the rated point of relief for coolant to enter the Expansion tank – not vent to atmosphere. -

Proper Water Heater Selection
Strategy Guideline: Proper Water Heater Selection M. Hoeschele, D. Springer, A. German, J. Staller, and Y. Zhang Alliance for Residential Building Innovation (ARBI) Revised April 2015 NOTICE This report was prepared as an account of work sponsored by an agency of the United States government. Neither the United States government nor any agency thereof, nor any of their employees, subcontractors, or affiliated partners makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States government or any agency thereof. Available electronically at http://www.osti.gov/bridge Available for a processing fee to U.S. Department of Energy and its contractors, in paper, from: U.S. Department of Energy Office of Scientific and Technical Information P.O. Box 62 Oak Ridge, TN 37831-0062 phone: 865.576.8401 fax: 865.576.5728 email: mailto:[email protected] Available for sale to the public, in paper, from: U.S. Department of Commerce National Technical Information Service 5285 Port Royal Road Springfield, VA 22161 phone: 800.553.6847 fax: 703.605.6900 email: [email protected] online ordering: http://www.ntis.gov/ordering.htm Printed on paper containing at least 50% wastepaper, including 20% postconsumer waste Strategy Guideline: Proper Water Heater Selection Prepared for: Building America Building Technologies Program Office of Energy Efficiency and Renewable Energy U.S. -

Boilermate Installation and Operation Instructions
BOILERMATE® INDIRECT-FIRED WATER HEATERS INSTALLATION & OPERATION INSTRUCTIONS Models WH (Mechanical Control & Digital Control) Models CH (Mechanical Control) NOTE: A properly sized thermal expansion tank is required and should be installed in accordance with the product installation manual. NOTE: The thermostat is adjusted to 120°F when shipped from the factory. NOTE: Inspect for shipping damage and notify freight carrier or store where purchased immediately if damage is present. To avoid risk of personal injury and property damage, if the product appears to be malfunctioning or shows signs of corrosion, call a qualified professional immediately. Current copies of the Product manual can be viewed at www.amtrol.com. Use proper safety equipment when installing. THIS IS THE SAFETY ALERT SYMBOL. IT IS USED TO ALERT YOU TO POTENTIAL PERSONAL INJURY AND OTHER HAZARDS. OBEY ALL SAFETY MESSAGES THAT FOLLOW THIS SYMBOL TO REDUCE THE RISK OF PERSONAL INJURY AS WELL AS PROPERTY DAMAGE. READ CAREFULLY THE PRODUCT FAILURE TO INSPECT THIS VALVE AS DIRECTED COULD RESULT INSTALLATION & OPERATION INSTRUCTIONS. IN UNSAFE TEMPERATURE OR PRESSURE BUILD-UP WHICH FAILURE TO FOLLOW THE INSTRUCTIONS AND WARNINGS IN THE CAN RESULT IN PRODUCT FAILURE, SERIOUS INJURY OR MANUAL MAY RESULT IN SERIOUS OR FATAL INJURY AND/OR DEATH AND/OR SEVERE PROPERTY DAMAGE AND VOID THE PROPERTY DAMAGE, AND WILL VOID THE PRODUCT WARRANTY. PRODUCT WARRANTY. THIS PRODUCT MUST BE INSTALLED BY A QUALIFIED Chlorine Aggressive Water: The water quality can PROFESSIONAL. FOLLOW ALL APPLICABLE LOCAL AND STATE significantly influence the life of this Product. You CODES AND REGULATIONS, IN THE ABSENCE OF SUCH CODES, should test for corrosive elements, acidity, total solids and other relevant FOLLOW THE CURRENT EDITIONS OF THE NATIONAL PLUMBING contaminants, including chlorine and treat your water appropriately to CODE AND NATIONAL ELECTRIC CODE, AS APPLICABLE.