Date: 1/9/2017 Question: Botulism Is an Uncommon Disorder Caused By
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chemical Threat Agents Call Poison Control 24/7 for Treatment Information 1.800.222.1222 Blood Nerve Blister Pulmonary Metals Toxins
CHEMICAL THREAT AGENTS CALL POISON CONTROL 24/7 FOR TREATMENT INFORMATION 1.800.222.1222 BLOOD NERVE BLISTER PULMONARY METALS TOXINS SYMPTOMS SYMPTOMS SYMPTOMS SYMPTOMS SYMPTOMS SYMPTOMS • Vertigo • Diarrhea, diaphoresis • Itching • Upper respiratory tract • Cough • Shock • Tachycardia • Urination • Erythema irritation • Metallic taste • Organ failure • Tachypnea • Miosis • Yellowish blisters • Rhinitis • CNS effects • Cyanosis • Bradycardia, bronchospasm • Flu-like symptoms • Coughing • Shortness of breath • Flu-like symptoms • Emesis • Delayed eye irritation • Choking • Flu-like symptoms • Nonspecific neurological • Lacrimation • Delayed pulmonary edema • Visual disturbances symptoms • Salivation, sweating INDICATIVE LAB TESTS INDICATIVE LAB TESTS INDICATIVE LAB TEST INDICATIVE LAB TESTS INDICATIVE LAB TESTS INDICATIVE LAB TESTS • Increased anion gap • Decreased cholinesterase • Thiodiglycol present in urine • Decreased pO2 • Proteinuria None Available • Metabolic acidosis • Increased anion gap • Decreased pCO2 • Renal assessment • Narrow pO2 difference • Metabolic acidosis • Arterial blood gas between arterial and venous • Chest radiography samples DEFINITIVE TEST DEFINITIVE TEST DEFINITIVE TEST DEFINITIVE TESTS DEFINITIVE TESTS • Blood cyanide levels • Urine nerve agent • Urine blister agent No definitive tests available • Blood metals panel • Urine ricinine metabolites metabolites • Urine metals panel • Urine abrine POTENTIAL AGENTS POTENTIAL AGENTS POTENTIAL AGENTS POTENTIAL AGENTS POTENTIAL AGENTS POTENTIAL AGENTS • Hydrogen Cyanide -

Theoretical Studies of Phenylmercury Carboxylates
TA Theoretical studies of Phenylmercury 2750 carboxylates 2010 This page is intentionally left blank Theoretical studies of Phenylmercury carboxylates Complexation Constants and Gas Phase Photo- Oxidation of Phenylmercurycarboxylates. Yizhen Tang and Claus Jørgen Nielsen CTCC, Department of Chemistry, University of Oslo This page is intentionally left blank Table of Contents Executive Summary ................................................................................................................ 1 1. Quantum chemistry studies on phenylmercurycarboxylates ..................................... 3 2. Results from quantum chemistry ................................................................................... 3 2.1 Structure of phenylmercury(II) carboxylates .............................................................. 4 2.2 Energies of complexation/dissociation ....................................................................... 9 2.3 Vertical excitation energies......................................................................................... 10 2.4 Vertical ionization energies ......................................................................................... 11 2.5 Summary of results from quantum chemistry calculations .................................... 11 3. Atmospheric fate and lifetimes of phenylmercurycarboxylates ............................... 12 3.1 Literature data............................................................................................................... 12 3.2 Estimation of the -

Stability of Dimethylmercury and Related Mercury-Containing Compounds with Respect to Selected Chemical Species Found in Aqueous Environment†
CROATICA CHEMICA ACTA CCACAA, ISSN 0011-1643, e-ISSN 1334-417X Croat. Chem. Acta 86 (4) (2013) 453–462. http://dx.doi.org/10.5562/cca2314 Original Scientific Article Stability of Dimethylmercury and Related Mercury-containing Compounds † with Respect to Selected Chemical Species Found in Aqueous Environment Laimutis Bytautas Department of Chemistry, Rice University, Houston, Texas 77005, USA, Present address: Galveston College, Department of Chemistry, 4015 Avenue Q, Galveston, TX, 77550, USA. (E-mail: [email protected]) RECEIVED JUNE 23, 2013; REVISED OCTOBER 3, 2013; ACCEPTED OCTOBER 4, 2013 Abstract. Dimethylmercury (CH3−Hg−CH3) and other Hg-containing compounds can be found in atmos- pheric and aqueous environments. These substances are highly toxic and pose a serious environmental and health hazard. Therefore, the understanding of chemical processes that affect the stability of these sub- stances is of great interest. The mercury-containing compounds can be detected in atmosphere, as well as soil and aqueous environments where, in addition to water molecules, numerous ionic species are abun- dant. In this study we explore the stability of several small, Hg-containing compounds with respect to wa- + ter molecules, hydronium (H3O ) ions as well as other small molecules/ions using density functional theo- ry and wave function quantum chemistry methods. It is found that the stability of such molecules, most + notably of dimethylmercury, can be strongly affected by the presence of the hydronium H3O ions. Alt- hough the present theoretical study represents gas phase results, it implies that pH level of a solution should be a major factor in determining the degree of abundance for dimethylmercury in aqueous envi- + ronment. -

Results of the Lake Michigan Mass Balance Study: Mercury Data Report
Results of the Lake Michigan Mass Balance Study: Mercury Data Report February 2004 U.S. Environmental Protection Agency Great Lakes National Program Office (G-17J) 77 West Jackson Boulevard Chicago, IL 60604 EPA 905 R-01-012 Results of the Lake Michigan Mass Balance Study: Mercury Data Report Prepared for: US EPA Great Lakes National Program Office 77 West Jackson Boulevard Chicago, Illinois 60604 Prepared by: Harry B. McCarty, Ph.D., Ken Miller, Robert N. Brent, Ph.D., and Judy Schofield DynCorp (a CSC Company) 6101 Stevenson Avenue Alexandria, Virginia 22304 and Ronald Rossmann, Ph.D. US EPA Office of Research and Development Large Lakes Research Station 9311 Groh Road Grosse Ile, Michigan 48138 February 2004 Acknowledgments This report was prepared under the direction of Glenn Warren, Project Officer, USEPA Great Lakes National Program Office; and Louis Blume, Work Assignment Manager and Quality Assurance Officer, USEPA Great Lakes National Program Office (GLNPO). The report was prepared by Harry B. McCarty, Ken Miller, Robert N. Brent, and Judy Schofield, with DynCorp’s Science and Engineering Programs, and Ronald Rossmann, USEPA Large Lakes Research Station, with significant contributions from the LMMB Principal Investigators for mercury and Molly Middlebrook, of DynCorp. GLNPO thanks these investigators and their associates for their technical support in project development and implementation. Ronald Rossmann wishes to thank Theresa Uscinowicz for assistance with collection, preparation, and analysis of the samples; special thanks to staff of the NOAA Great Lakes Environmental Research Laboratory, University of Wisconsin-Milwaukee Great Lakes Water Institute Center for Great Lakes Studies, USEPA Great Lakes National Program, and USEPA Mid-Continent Ecology Division for collection of the samples. -

Redalyc.Significance of Specific Igg Against Sensitizing Antigens In
Revista Portuguesa de Pneumología ISSN: 0873-2159 [email protected] Sociedade Portuguesa de Pneumologia Portugal Sterclova, M.; Vasakova, M.; Metlicka, M. Significance of specific IgG against sensitizing antigens in extrinsic allergic alveolitis: Serological methods in EAA Revista Portuguesa de Pneumología, vol. 17, núm. 6, noviembre-diciembre, 2011, pp. 253-259 Sociedade Portuguesa de Pneumologia Lisboa, Portugal Available in: http://www.redalyc.org/articulo.oa?id=169722769004 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Documento descarregado de http://www.elsevier.pt el 21/05/2012. Cópia para uso pessoal, está totalmente proibida a transmissão deste documento por qualquer meio ou forma. Rev Port Pneumol. 2011;17(6):253---259 www.revportpneumol.org ORIGINAL ARTICLE Significance of specific IgG against sensitizing antigens in extrinsic allergic alveolitis: Serological methods in EAA M. Sterclova a,∗, M. Vasakova b, M. Metlicka b a Department of Respiratory Diseases, Thomayer’s University Hospital, Videnska, Prague, Czech Republic b Department of Immunology, Thomayer’s University Hospital, Videnska, Prague, Czech Republic Received 23 December 2010; accepted 20 June 2011 Available online 16 September 2011 KEYWORDS Abstract The aim of our study is to find differences in IgG in sera of potentially exposed and Aeroallergen; nonexposed individuals and to detect differences in concentrations of specific serum IgG among Hypersensitivity subjects with and without EAA. pneumonitis; Seventy-two patients being followed for suspected interstitial lung disease were included. -

Notes from Rao 2/13/2007 2:07:37 PM
Environmental Assessment of Sediments and Water in Bayou Grande, Pensacola, FL. Dr. Carl J. Mohrherr Center for Environmental Diagnostics and Bioremediation University of West Florida Dr. Johan Liebens Department of Environmental Studies University of West Florida Dr. K. Ranga Rao Center for Environmental Diagnostics and Bioremediation University of West Florida June 26, 2008 FOREWORD This study is a component of the "Assessment of Environmental Pollution and Community Health in Northwest Florida" supported by a USEPA Cooperative Agreement award X-9745502 to The University of West Florida (Project Director: Dr. K. Ranga Rao). The contents of this report are solely the responsibility of the authors and do not necessarily represent the official views of the USEPA. The study was undertaken because of the increasing concern for environmental pollution and potential impacts on human health in Northwest Florida. It was designed to assess environmental impacts of toxic pollutants in Bayou Grande. Kristal Flanders managed the spatial databases for the project and drafted the maps. Her assistance has been invaluable. Chris Carlton-Franco, Brandon Jarvis, Guy Allard, and Danielle Peterson helped with the fieldwork and some laboratory procedures. Creative Commons License This work is licensed under a Creative Commons Attribution- NonCommercial- NoDerivatives 4.0 International License. i TABLE OF CONTENTS I INTRODUCTION........................................................................................................... 1 II STUDY AREA............................................................................................................. -

Pyrophoric Materials
Appendix A PYROPHORIC MATERIALS Pyrophoric materials react with air, or with moisture in air. Typical reactions which occur are oxidation and hydrolysis, and the heat generated by the reactions may ignite the chemical. In some cases, these reactions liberate flammable gases which makes ignition a certainty and explosion a real possibility. Examples of pyrophoric materials are shown below. (List may not be complete) (a) Pyrophoric alkyl metals and derivatives Groups Dodecacarbonyltetracobalt Silver sulphide Dialkytzincs Dodecacarbonyltriiron Sodium disulphide Diplumbanes Hexacarbonylchromium Sodium polysulphide Trialkylaluminiums Hexacarbonylmolybdenum Sodium sulphide Trialkylbismuths Hexacarbonyltungsten Tin (II) sulphide Nonacarbonyldiiron Tin (IV) sulphide Compounds Octacarbonyldicobalt Titanium (IV) sulphide Bis-dimethylstibinyl oxide Pentacarbonyliron Uranium (IV) sulphide Bis(dimethylthallium) acetylide Tetracarbonylnickel Butyllithium (e) Pyrophoric alkyl non-metals Diethylberyllium (c) Pyrophoric metals (finely divided state) Bis-(dibutylborino) acetylene Bis-dimethylarsinyl oxide Diethylcadmium Caesium Rubidium Bis-dimethylarsinyl sulphide Diethylmagnesium Calcium Sodium Bis-trimethylsilyl oxide Diethylzinc Cerium Tantalum Dibutyl-3-methyl-3-buten-1-Yniborane Diisopropylberyllium Chromium Thorium Diethoxydimethylsilane Dimethylberyllium Cobalt Titanium Diethylmethylphosphine Dimethylbismuth chloride Hafnium Uranium Ethyldimthylphosphine Dimethylcadmium Iridium Zirconium Tetraethyldiarsine Dimethylmagnesium Iron Tetramethyldiarsine -

PROVISIONAL PEER REVIEWED TOXICITY VALUES for DIMETHYLMERCURY (CASRN 593-74-8) Derivation of Subchronic and Chronic Oral Rfds
EPA/690/R-04/005F l Final 12-01-2004 Provisional Peer Reviewed Toxicity Values for Dimethylmercury (CASRN 593-74-8) Derivation of Subchronic and Chronic Oral RfDs Superfund Health Risk Technical Support Center National Center for Environmental Assessment Office of Research and Development U.S. Environmental Protection Agency Cincinnati, OH 45268 Acronyms and Abbreviations bw body weight cc cubic centimeters CD Caesarean Delivered CERCLA Comprehensive Environmental Response, Compensation and Liability Act of 1980 CNS central nervous system cu.m cubic meter DWEL Drinking Water Equivalent Level FEL frank-effect level FIFRA Federal Insecticide, Fungicide, and Rodenticide Act g grams GI gastrointestinal HEC human equivalent concentration Hgb hemoglobin i.m. intramuscular i.p. intraperitoneal i.v. intravenous IRIS Integrated Risk Information System IUR inhalation unit risk kg kilogram L liter LEL lowest-effect level LOAEL lowest-observed-adverse-effect level LOAEL(ADJ) LOAEL adjusted to continuous exposure duration LOAEL(HEC) LOAEL adjusted for dosimetric differences across species to a human m meter MCL maximum contaminant level MCLG maximum contaminant level goal MF modifying factor mg milligram mg/kg milligrams per kilogram mg/L milligrams per liter MRL minimal risk level i MTD maximum tolerated dose MTL median threshold limit NAAQS National Ambient Air Quality Standards NOAEL no-observed-adverse-effect level NOAEL(ADJ) NOAEL adjusted to continuous exposure duration NOAEL(HEC) NOAEL adjusted for dosimetric differences across species to a -

Statistical Analysis Plan
Non-Interventional Study Protocol Study Code << DXXXRXXX >> Version V1.4 Date 14 July 2017 Decline In lung-function Among Patients with chronic obstructive Lung disease On maintenance therapy (DIAPLO) An observational study evaluating the benefits of early intervention with maintenance therapies to prevent or slow down rapid lung function decline in patients who are at high risk at the time of COPD diagnosis in the combined Optimum Patient Care Research Database and Clinical Practice Research Datalink databases TITLE PAGE Non-Interventional Study Protocol Study Code << DXXXRXXX >> Version 14 July 2017 Date 14 July 2017 TABLE OF CONTENTS PAGE TITLE PAGE ........................................................................................................... 1 TABLE OF CONTENTS ......................................................................................... 2 LIST OF ABBREVIATIONS .................................................................................. 5 RESPONSIBLE PARTIES ...................................................................................... 6 PROTOCOL SYNOPSIS DIAPLO STUDY ........................................................... 7 AMENDMENT HISTORY ................................................................................... 12 MILESTONES ....................................................................................................... 13 1. BACKGROUND AND RATIONALE .................................................................. 14 1.1 Background ........................................................................................................... -

Date: 1/9/2017 Question: Botulism Is an Uncommon Disorder Caused By
6728 Old McLean Village Drive, McLean, VA 22101 Tel: 571.488.6000 Fax: 703.556.8729 www.clintox.org Date: 1/9/2017 Question: Botulism is an uncommon disorder caused by toxins produced by Clostridium botulinum. Seven subtypes of botulinum toxin exist (subtypes A, B, C, D, E, F and G). Which subtypes have been noted to cause human disease and which ones have been reported to cause infant botulism specifically in the United States? Answer: According to the cited reference “Only subtypes A, B, E and F cause disease in humans, and almost all cases of infant botulism in the United States are caused by subtypes A and B. Botulinum-like toxins E and F are produced by Clostridium baratii and Clostridium butyricum and are only rarely implicated in infant botulism” (Rosow RK and Strober JB. Infant botulism: Review and clinical update. 2015 Pediatr Neurol 52: 487-492) Date: 1/10/2017 Question: A variety of clinical forms of botulism have been recognized. These include wound botulism, food borne botulism, and infant botulism. What is the most common form of botulism reported in the United States? Answer: According to the cited reference, “In the United States, infant botulism is by far the most common form [of botulism], constituting approximately 65% of reported botulism cases per year. Outside the United States, infant botulism is less common.” (Rosow RK and Strober JB. Infant botulism: Review and clinical update. 2015 Pediatr Neurol 52: 487-492) Date: 1/11/2017 Question: Which foodborne pathogen accounts for approximately 20 percent of bacterial meningitis in individuals older than 60 years of age and has been associated with unpasteurized milk and soft cheese ingestion? Answer: According to the cited reference, “Listeria monocytogenes, a gram-positive rod, is a foodborne pathogen with a tropism for the central nervous system. -

High Hazard Chemical Policy
Environmental Health & Safety Policy Manual Issue Date: 2/23/2011 Policy # EHS-200.09 High Hazard Chemical Policy 1.0 PURPOSE: To minimize hazardous exposures to high hazard chemicals which include select carcinogens, reproductive/developmental toxins, chemicals that have a high degree of toxicity. 2.0 SCOPE: The procedures provide guidance to all LSUHSC personnel who work with high hazard chemicals. 3.0 REPONSIBILITIES: 3.1 Environmental Health and Safety (EH&S) shall: • Provide technical assistance with the proper handling and safe disposal of high hazard chemicals. • Maintain a list of high hazard chemicals used at LSUHSC, see Appendix A. • Conduct exposure assessments and evaluate exposure control measures as necessary. Maintain employee exposure records. • Provide emergency response for chemical spills. 3.2 Principle Investigator (PI) /Supervisor shall: • Develop and implement a laboratory specific standard operation plan for high hazard chemical use per OSHA 29CFR 1910.1450 (e)(3)(i); Occupational Exposure to Hazardous Chemicals in Laboratories. • Notify EH&S of the addition of a high hazard chemical not previously used in the laboratory. • Ensure personnel are trained on specific chemical hazards present in the lab. • Maintain Material Safety Data Sheets (MSDS) for all chemicals, either on the computer hard drive or in hard copy. • Coordinate the provision of medical examinations, exposure monitoring and recordkeeping as required. 3.3 Employees: • Complete all necessary training before performing any work. • Observe all safety -
This Poster I It's Designed Large the Placeho Formatted Fo Placeholder
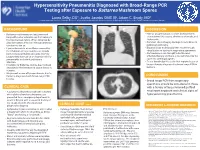
Hypersensitivity Pneumonitis Diagnosed with Broad-Range PCR Testing after Exposure to Battarrea Mushroom Spores • Laura Selby, DO1, Justin Jacobs OMS III2, Adam C. Brady, MD1 Printing: 1. Samaritan Health Services, Corvallis, Oregon 2. Western University of Health Sciences College of Osteopathic Medicine of the Pacific Northwest , Lebanon, Oregon This poster is 48” wide by 36” high. It’s designed to be printed on a BACKGROUND DISCUSSION large • Battarrea mushrooms are long-stemmed • Human lycoperdonosis is a rare disease that is fungi with a characteristic cap that belong to characterized by nausea, shortness of breath, and the Agaricaceae family. When disturbed by tachycardia. physical contact, they can release numerous • Common chest imaging findings include bilateral Customizing the Content: conidia into the air. pulmonary infiltrates. • Lycoperdonosis is a rare illness caused by • Diagnosis has traditionally been made through The placeholders in this the inhalation of large numbers of conidia visualization of spores in respiratory specimens. from certain puffball mushrooms (namely • Corticosteroids are thought to be the most formatted for you. Lycoperdon) that can cause hypersensitivity effective therapy and there is no definitive role for placeholders to add text, or click pneumonitis and mimic pulmonary systemic antifungal agents. infection. • To our knowledge this is the first reported case of an icon to add a table, chart, • Inhalation of Battarrea conidia has not been lycoperdonosis diagnosed by broad range PCR in described in the literature to cause illness in humans. SmartArt graphic, picture or humans. multimedia file. • We present a case of lycoperdonosis due to Battarea, diagnosed with broad-range PCR CONCLUSION T testing.