Statistical Analysis Plan
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nutrition Options in Short-Bowel Syndrome Upmcphysicianresources.Com/GI Instructions: Services
In This Issue 1 Nutrition Options in Short-Bowel Syndrome SPRING 2017 Division of Gastroenterology, 3 Gastric Carcinoids with Duodenal Ulcers Hepatology, and Nutrition 4 Living Donor Liver Transplant (LDLT) 6 PancreasFest 2017 / Honors and Awards 7 Pittsburgh Gut Club 8 What Is This? Nutrition Options in Short-Bowel Syndrome By David G. Binion, MD, and Zachary Zator, MD Intestinal transplantation is an option for select patients with short-bowel syndrome- associated intestinal failure (SBS-IF) who fail or do not tolerate nutritional rehabilitation. There are a range of factors to consider in the nutritional management of patients before and after intestinal transplantation. SBS-IF can be defined as the inability to maintain proper nutritional balance — including of proteins, electrolytes, macronutrients, micronutrients, and fluids — while adhering to a conventional diet in the face of an anatomically or functionally limited gut surface. The ideal management of patients with SBS-IF involves a multidisciplinary team of gastro enterologists, nurses, dietitians, pharmacists, and surgeons. Pharmacotherapeutic agents aimed at minimizing fluid losses have been routinely employed to support these patients. For instance, antidiarrheal agents, such as loperamide or diphenoxylate, are used alongside proton pump inhibitors. Somatostatin analogs, like octreotide, inhibit gastrointestinal secretions from the stomach, pancreas, and intestines and have been proven beneficial in the past. However, their role can be limited, as somatostatin can actually -
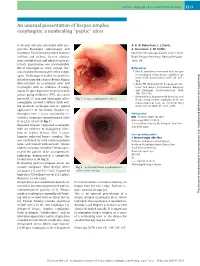
An Unusual Presentation of Herpes Simplex Esophagitis: a Nonhealing “Peptic” Ulcer
UCTN – Unusual cases and technical notes E213 An unusual presentation of herpes simplex esophagitis: a nonhealing “peptic” ulcer A 58-year-old man presented with pro- A. G. N. Robertson, L. J. Dunn, gressive dysphagia, odynophagia, and A. Immanuel, S. M. Griffin heartburn. Past history included diabetes Northern Oesophago-Gastric Cancer Unit, mellitus and asthma. Current medica- Royal Victoria Infirmary, Newcastle Upon tions included oral and inhaled corticos- Tyne, UK teroids. Examination was unremarkable. Blood investigations were normal. Bar- References ium swallow demonstrated reflux esoph- 1 Fass R. Symptom assessment tools for gas- agitis. Endoscopy revealed circumferen- troesophageal reflux disease (GERD) treat- ment. J Clin Gastroenterol 2007; 41: 437– tial ulceration and a hiatus hernia. Biopsy 444 demonstrated an esophageal ulcer and 2 Baehr PH, McDonald GB. Esophageal infec- esophagitis with no evidence of malig- tions: risk factors, presentation, diagnosis, nancy. Despite high-dose treatment with and treatment. Gastroenterology 1994; proton pump inhibitors (PPI), ulceration 106: 509 – 532 3 Ramanathan J, Rammouni M, Baran J Jr et al. persisted. CT scan and endoscopic ultra- Fig. 1 Herpes esophageal ulceration. Herpes simplex virus esophagitis in the im- sonography showed a diffuse thick-wal- munocompetent host: an overview. Am J led proximal esophagus but no typical Gastroenterol 2000; 95: 2171– 2176 appearances of carcinoma. Regular en- doscopies over 2 years consistently re- Bibliography vealed a suspicious circumferential ulcer DOI 10.1055/s-0029-1214687 from 22 to 25 cm (l" Fig. 1). Endoscopy 2009; 41: E213 Georg Thieme Verlag KG Stuttgart · New York · Repeated biopsies suggested esophagitis ISSN 0013-726X with no evidence of malignancy, infec- tion, or Crohn’s disease. -

Herpetic Esophagitis: a Diagnostic Challenge in Immunocompromised Patients
0(X)2-9270/86/8l()4-0246 THE AMERICAN JOURNAL OF GASTROENTEROLOGY Vol.81, No.4, 1986 Copyright © 1986 by Am. Coll. of Gastroenterology Primed in US.A. Herpetic Esophagitis: A Diagnostic Challenge in Immunocompromised Patients Farooq P. Agha, M.D., F.A.C.G., Horchang H. Lee, M.D., M.P.H., and Timothy T. Nostrant, M.D. Department of Radiology, and Internal Medicine-Division of Gastroenterology. University of Michigan Hospitals and Medical Center, Ann Arbor. Michigan Viral esophageal infection is eommon in immunocom- these patients to infections were; diffuse histiocytic promised patients. Twelve patients wi(b esopbagitis lymphoma in three, chronic granulocytic leukemia in secondary to herpes viruses are described. Odyno- two, diabetes mellitus in three, prolonged steroid ther- phagia, dysphagia, and gastrointestinal bleeding were apy in two, extensive burns in one, renal transplanta- the most eommon symptoms. Multiple infections par- tion in two, diffuse carcinomatosis in one, and acquired tieularly with Candida were present in three of the 12 immunedeficiency syndrome in one patient. All pa- cases (25%). Typical "volcano ulcers" at endoseopy and tients were immunosuppressed and usually multiple discrete diffusely scattered shallow uleers seen on dou- predisposing factors were responsible. All patients with ble contrast esophagram are highly suggestive of her- hematological malignancy had received extensive petic esophagitis. Single contrast esophagram plays no chemotherapy before the onset of herpetic infection. specific role in the diagnosis of herpetie esophagitis. An The pertinent clinical data on these 12 patients are analysis of elinieal, endoscopic, radiologieal, and path- summarized in Table I. ologieal features is presented. All patients were symptomatic at the time of diag- nosis. -

Nova Et Vetera .Of the Times Recognized the Necessity of Direct Contact with the Hellenic Writings
SEPT. 1936 LINACRE AND THE SCHOLAR-PHYSICIANS OF OXFORD <THEBRITISH 550 12, MNEDICAL JOURNAL I These conditions could not endure. Arabian-taught medicine was scholastic and sterile. Powerful thinkers Nova et Vetera .of the times recognized the necessity of direct contact with the Hellenic writings. John Basingstoke, an Oxford man, travelled to Greece, and there learnt Greck THOMAS LINACRE AND THE FIRST from a learned Athenian woman, Constantina. He re- soon * turned to England with Greek manuscripts, and was SCHOLAR-PHYSICIANS OF OXFORD in contact with the great churchman and scholar Robert BY Grosseteste (1175-1253). Grosseteste's life is in large part bound up with Oxford, where he was educated. A. P. CAWADIAS, O.B.E., M.D., F.R.C.P. Wishing to increase his knowledge of true science he PHYSICIAN TO TIIE ST. JOHN CLINIC AND INSTITUTE OF PHYSICAL MEDICINE studied Greek not only at second hand in Paris but at Oxford with a native Greek, Nicolas or Elicheros, and began to translate Greek authors, unfortunately not im- Thomas Linacre and Leonicenus were the greatest portant writers. His friend and Oxford contemporary, physicians of the early Renaissance. Around the former Roger Bacon, the Doctor mirabilis, with the courage radiated a group of other eminent physicians who, with and energy which characterized his whole life, pointed the exception of Caius, were all of Oxford. The studies out that the knowledge received from the Arabian writers and medical preparation of Linacre belong to the last was imperfect because of faulty translation, and he quarter of the fifteenth the period of active century; blamed the professors for not learning Greek so as to life, as in the case of the other great Oxford scholar- be able to read Aristotle and other writers in the original. -

Medical School of Maine Catalogue (1892)
Bowdoin College Bowdoin Digital Commons Bowdoin College Catalogues 1-1-1892 Bowdoin College - Medical School of Maine Catalogue (1892) Bowdoin College Follow this and additional works at: https://digitalcommons.bowdoin.edu/course-catalogues Recommended Citation Bowdoin College, "Bowdoin College - Medical School of Maine Catalogue (1892)" (1892). Bowdoin College Catalogues. 139. https://digitalcommons.bowdoin.edu/course-catalogues/139 This Book is brought to you for free and open access by Bowdoin Digital Commons. It has been accepted for inclusion in Bowdoin College Catalogues by an authorized administrator of Bowdoin Digital Commons. For more information, please contact [email protected]. j. DEC 4 1909 &A 3ft £WfCK t *i fyEDlWE ggf?0OE OP fpip. By 1892. : MEDICAL SCHOOL OF MAINE AT BOWB0IN G0LLESE. 72nd Course. COMMENCING FEBRUARY 4TH, 1892. BRUNSWICK TELEGRAPH JOB PRESS, 18 31. FACULTY. REV. WILLIAM DeWITT HYDE, D. D., President ALFRED MITCHELL, A. M., M. D., Secretary ISRAEL THORNDIKE DANA, A. M., M. D., Pathology and Practice ALFRED MITCHELL, A. M., M. D., Obstetrics and Diseases of Women and Children FREDERIC HENRY GERRISH, A. M., M. D., Anatomy FRANKLIN CLEMENT ROBINSON, A. M., Chemistry STEPHEN HOLMES WEEKS, A. M., M. D., Surgery and Clinical Surgery CHARLES OLIVER HUNT, A. M., M., D., Materia Medica and Therapeutics Hon. LUCILIUS ALONZO EMERY, A. M., Medical Jurisprudence CHARLES DENNISON SMITH, A. M., M. D., Physiology and Public Hygiene EVERETT THORNTON NE ALEY, M. D., Demonstrator of Histology ADDISON SANFORD THAYER, A. B., M. D., Demonstrator of Anatomy Hon. WILLIAM LeBARON PUTNAM, LL. D., ) From the Board Hon. JOSEPH TITCOMB., C of Trustees. -

Herpes and Cytomegalovirus Esophagitis
E242 UCTN – Unusual cases and technical notes Herpes and cytomegalovirus esophagitis Fig. 1 Upper gastrointestinal endoscopy in a 46-year-old transplant recipient who had recently been treated with high doses of steroids showing ulcerated mucosa in: a the mid-esophagus; b,c the upper esophagus. A 46-year-old man who underwent a liver Fig. 2 Histological transplant in 2001 for fulminant hepatitis appearance of the of unknown etiology was diagnosed with esophageal ulcers a liver non-Hodgkin lymphoma (post- revealing: a herpes transplant lymphoproliferative disease) simplex virus (HSV) in 2011. Some months later, he developed inclusions within the esophageal squamous an acute hepatocellular rejection that was cells; b cytomegalovirus treated with high doses of steroids. (CMV)-infected cells by The patient was admitted because of fever immunohistochemical and severe odynophagia that was hinder- staining; c HSV-infected ing oral intake. He had multiple painful cells by immunohisto- ulcers on his tongue, palate, and oral mu- chemical staining. cosa. Upper gastrointestinal endoscopy revealed large superficial, circumferential ulcers with well-defined margins and yel- low exudate in the mid and upper esoph- agus (●" Fig.1). Biopsies taken from the ulcer base and borders confirmed herpes simplex virus (HSV) and cytomegalovirus Esophageal ulcers due to CMV are typical- A. Albuquerque1, H. Cardoso1,2, (CMV) co-infection (●" Fig. 2). Polymerase ly large, shallow, solitary or multiple, and A. Ribeiro1, E. Rios3, R. Silva3, chain reaction (PCR) of the esophageal located in the mid or distal esophagus [3]. J. Magalhães3, G. Macedo1,2 mucosa for HSV and CMV DNA was posi- In HSV esophagitis, the morphology de- 1 Gastroenterology Department, Hospital tive. -

Simultaneous Candida Albicans and Herpes Simplex Virus Type 2
Reminder of important clinical lesson BMJ Case Rep: first published as 10.1136/bcr-2019-230410 on 15 August 2019. Downloaded from Case report Simultaneous candida albicans and herpes simplex virus type 2 esophagitis in a renal transplant recipient Imran Gani, 1 Vatsalya Kosuru,2 Muhammad Saleem,2 Rajan Kapoor1 1Nephrology, Hypertension and SUMMARY episode of biopsy-proven, borderline, acute cellular Transplant Medicine, Augusta Renal transplant recipients are prone to opportunistic rejection in the second month after transplant that University Health, Augusta, infections due to iatrogenic immunosuppression. responded well to pulse steroids with normalisation Georgia, USA Infectious esophagitis can present as an opportunistic of renal function. 2Internal Medicine, Augusta infection in the post-transplant period. Common Nine months after her kidney transplantation she University Health System, Augusta, Georgia, USA pathogens are candida, herpes simplex virus (HSV) presented with sore throat, dysphagia, odynophagia and cytomegalovirus (CMV). Having a dual infection and fever. Her physical examination was significant Correspondence to is uncommon and the diagnoses can be missed at for oropharyngeal thrush, white tonsillar exudates, Dr Imran Gani, initial presentation. Our patient, a 29-year-old African- pharyngeal erythema and enlarged palatine tonsils. igani@ augusta. edu American woman, status post deceased-donor-kidney Blood work showed leukocytosis and acute kidney transplant presented with difficulty and pain in injury (AKI). She was admitted to the hospital for Accepted 22 July 2019 swallowing with clinical features suggestive of candida intravenous fluids and intravenous fluconazole esophagitis, confirmed by fungal culture. She did not therapy for severe oropharyngeal candidiasis and get better with antifungal treatment. On further testing, high suspicion of esophageal candidiasis. -

La Salle College Magazine February 1957 La Salle University
La Salle University La Salle University Digital Commons La Salle Magazine University Publications 2-1957 La Salle College Magazine February 1957 La Salle University Follow this and additional works at: http://digitalcommons.lasalle.edu/lasalle_magazine Recommended Citation La Salle University, "La Salle College Magazine February 1957" (1957). La Salle Magazine. 202. http://digitalcommons.lasalle.edu/lasalle_magazine/202 This Book is brought to you for free and open access by the University Publications at La Salle University Digital Commons. It has been accepted for inclusion in La Salle Magazine by an authorized administrator of La Salle University Digital Commons. For more information, please contact [email protected]. BROTHER E. ^ ANISLAUS, F. S. C. SILVER JU 2ALARIAN '-.',, '-,..-.. '''•• ,; '--'-'"' •''; V-,' Digitized by the Internet Archive in 2011 with funding from LYRASIS members and Sloan Foundation http://www.archive.org/details/lasalle121957unse William G. Snyder '50 Editor l^>a SaLie John L. McCloskey '48 VOLUME NUMBER 2 Director of Alumni Cover Story The cover of this number of our magazine fittingly brings to our attention the likeness of Brother Stanislaus, President of our College, who is celebrating this month his twenty-fifth anni- versary as a Brother of the Christian Schools. The Alumni, in felicitating the jubilarian on reaching L. Thomas Reifsteck '51 the twenty-fifth milestone in his religious career, are not unmindful that much of Brother's Director of Placement quarter century of service, both as teacher and as administrator, have been spent here at La Salle. Their recollection of Brother Stanislaus is always associated with the idea of progress. Brother's aim as a teacher, first and always, was the progress of his students; as an administrator, he has identified himself wholeheartedly with the development of La Salle. -

The Psukhē in and Behind Clement of Alexandria's Paedagogus
University of Pennsylvania ScholarlyCommons Publicly Accessible Penn Dissertations 2016 Psukhai That Matter: The Psukhē in and Behind Clement of Alexandria’s Paedagogus Phillip Jay Webster University of Pennsylvania, [email protected] Follow this and additional works at: https://repository.upenn.edu/edissertations Part of the Ancient History, Greek and Roman through Late Antiquity Commons, and the Religion Commons Recommended Citation Webster, Phillip Jay, "Psukhai That Matter: The Psukhē in and Behind Clement of Alexandria’s Paedagogus" (2016). Publicly Accessible Penn Dissertations. 2088. https://repository.upenn.edu/edissertations/2088 This paper is posted at ScholarlyCommons. https://repository.upenn.edu/edissertations/2088 For more information, please contact [email protected]. Psukhai That Matter: The Psukhē in and Behind Clement of Alexandria’s Paedagogus Abstract This dissertation aims to investigate the ideology and mechanics of the ancient soul’s materiality as witnessed in Clement of Alexandria’s late second- or early third-century work, the Paedagogus. I focus on four ways in which Clement refers to the soul: (1) as an entity in need of punishment and healing, (2) as vulnerable to substances and the activities of the body, (3) as made visible through the body’s appearance, and (4) as an internal moral-core. Through the lens of the Paedagogus, this dissertation introduces recent theoretical work on “materiality” and “the body,” especially as developed in gender studies, into the broad scholarly conversation about the ancient soul. In the process, it shows how Clement uses the interactions between the ancient soul and the ancient body in his attempt to produce and police Christian subjects. -

Herpes Simplex Virus and the Alimentary Tract
Herpes Simplex Virus and the Alimentary Tract Eric A. Lavery, MD , and Walter J. Coyle , MD Corresponding author infections of the gastrointestinal tract in both immuno- Walter J. Coyle, MD Division of Gastroenterology and Hepatology Scripps Clinic compromised and immunocompetent patients. Torrey Pines, 10666 North Torrey Pines Road, N203, La Jolla, CA 92037, USA. E-mail: [email protected] Background Current Gastroenterology Reports 2008, 10: 417– 423 HSV is a member of the Herpesviridae family of viruses, Current Medicine Group LLC ISSN 1522-8037 which also includes varicella zoster virus (VZV), cyto- Copyright © 2008 by Current Medicine Group LLC megalovirus (CMV), Epstein-Barr virus (EBV), and human herpesvirus (HHV) 6, 7, and 8 [ 2• ]. Members of this family contain linear, double-stranded DNA within a Herpes simplex virus (HSV) infection is well known as protein capsid, which is surrounded by a tegument and an a sexually transmitted disease. However, relatively little outer glycoprotein layer [ 2• , 3•• ]. HSV-1 and HSV-2 have has been published concerning the presentations and 70% genomic homology but tend to affect different areas treatment of HSV infection within the gastrointestinal of the body. HSV-1 tends to cause most oral and esopha- tract, where HSV most commonly affects the esophagus geal herpetic lesions; it is commonly acquired during in both immunocompromised and immunocompetent childhood, though it has been associated with proctitis in patients. HSV proctitis is not uncommon and occurs a minority of cases. HSV-1 is primarily transmitted via primarily in males having sex with males. In patients oral secretions and has a higher seroprevalence in lower with normal immune systems, gastrointestinal HSV socioeconomic communities. -

1860 CENSUS of BALTIMORE CITY
1860 CENSUS of BALTIMORE CITY *#*»#»/########»####»#»###»#»*#»###»^»##»»»##»^^*^^»#^^^#^#^^^#****#**^^^»»##»»###»»»»»##»»»»»»»»»»#»j#»»# Volume Two Published by FAMILY LINE PUBLICATIONS Rear 63 East Main Street Westminster, Maryland 21157 GENEALOGY/LOCAL HISTORY/EARLY MAPS of Maryland, Delaware, Washington, D.C. & Pennsylvania Also available 1860 Census of Baltimore City, Wards I & II Send for free catalog. Copyright 1989 by Martha & Bill Reamy Printed in the U.S.A. Published 1989 by FAMILY LINE PUBLICATIONS INTRODUCTION Every effort has been made to achieve accuracy in this project, but interpreting the enumerator's hand-written material has posed problems. As an aid to deciphering many poorly written and misspelled names, the "Wood's Baltimore City Directory, 1861" was consulted. When an entry was found in the Directory where the first name and occupation agreed with the Census listing and the surname appears to be similar, the City Directory spelling was added in brackets in the text and added to the index. The original enumeration at the National Archives was consulted for all proofreading. The enumerator took great liberties in the spelling of surnames. Sometimes when an entry for a household carried over from one page to another the enumerator changed the spelling of the surname. We have retained both spellings in this book. Surnames were occasionally spelled phonetically, e.g. the name written as Knobloch in the Baltimore City Directory appears as Noblock in the census. The user is cautioned to check the index for all possible variations. In some sections it is obvious that the information was transcribed from the original record column by column rather than across the page, line by line, with frequent misalignment of the data on a particular line. -

Etiology, Diagnosis and Treatment of Infectious Esophagitis
Review paper Etiology, diagnosis and treatment of infectious esophagitis Mariusz Rosołowski1, Maciej Kierzkiewicz2 1Department of Gastroenterology and Internal Medicine, Medical University of Bialystok, Poland 22nd Department of Internal Medicine and Gastroenterology, Międzyleski Specialist Hospital, Warsaw, Poland Prz Gastroenterol 2013; 8 (6): 333–337 DOI: 10.5114/pg.2013.39914 Key words: infectious esophagitis, impaired immunity, Candida, herpes simplex virus, cytomegalovirus, acquired immunodeficiency syndrome. Address for correspondence: Mariusz Rosołowski MD, PhD, Department of Gastroenterology and Internal Medicine, Medical University of Bialystok, 26a M. Skłodowska-Curie St, 15-276 Bialystok, Poland, e-mail: [email protected] Abstract Infectious esophagitis may be caused by fungal, viral, bacterial or even parasitic agents. Risk factors include antibiotics and steroids use, chemotherapy and/or radiation therapy, malignancies and immunodeficiency syndromes including acquired immu- nodeficiency syndrome. Acute onset of symptoms such as dysphagia and odynophagia is typical. It can coexist with heartburn, retrosternal discomfort, nausea and vomiting. Abdominal pain, anorexia, weight loss and even cough are present sometimes. Infectious esophagitis is predominantly caused by Candida species. Other important causes include cytomegalovirus and herpes simplex virus infection. Candida-induced esophagitis when patients try to wear their dentures. Interestingly, many patients are asymptomatic. Esophagitis is predominantly caused