Herpes Esophagitis: a Comprehensive Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Statistical Analysis Plan
Title: Clinical effectiveness and safety of vedolizumab intravenous in real world clinical practice in ulcerative colitis Korean patients: a multicenter postmarketing observational study NCT Number: NCT03535649 SAP Approve Date: 03 DEC 2018 Certain information within this Statistical Analysis Plan has been redacted (ie, specific content is masked irreversibly from view with a black/blue bar) to protect either personally identifiable (PPD) information or company confidential information (CCI). This may include, but is not limited to, redaction of the following: Named persons or organizations associated with the study. Proprietary information, such as scales or coding systems, which are considered confidential information under prior agreements with license holder. Other information as needed to protect confidentiality of Takeda or partners, personal information, or to otherwise protect the integrity of the clinical study. CCI Statistical Analysis Plan Page 1 of 60 Statistical Analysis Plan STUDY ID: VEDOLIZUMAB-5045 TITLE: C LINICAL EFFECTIVENESS AND SAFETY OF VEDOLIZUMAB INTRAVENOUS IN REAL WORLD CLINICAL PRACTICE IN ULCERATIVE COLITIS KOREAN PATIENTS: A MULTICENTER POST-MARKETING OBSERVATIONAL STUDY SHORT TITLE: VEDOLIZUMAB IN ULCERATIVE COLITIS KOREAN PATIENTS Prepared for: Takeda Pharmaceuticals Korea Co., Ltd. PPD AUTHOR: VERSION NUMBER AND DATE: V2.0; 03 DEC 2018 Property of Takeda: For non-commercial use only and subject to the applicable Terms of Use Document: Takeda_SAP_Vedolizumab-5045_v2.0_20181203.docx Author: PPD Version -

16. Questions and Answers
16. Questions and Answers 1. Which of the following is not associated with esophageal webs? A. Plummer-Vinson syndrome B. Epidermolysis bullosa C. Lupus D. Psoriasis E. Stevens-Johnson syndrome 2. An 11 year old boy complains that occasionally a bite of hotdog “gives mild pressing pain in his chest” and that “it takes a while before he can take another bite.” If it happens again, he discards the hotdog but sometimes he can finish it. The most helpful diagnostic information would come from A. Family history of Schatzki rings B. Eosinophil counts C. UGI D. Time-phased MRI E. Technetium 99 salivagram 3. 12 year old boy previously healthy with one-month history of difficulty swallowing both solid and liquids. He sometimes complains food is getting stuck in his retrosternal area after swallowing. His weight decreased approximately 5% from last year. He denies vomiting, choking, gagging, drooling, pain during swallowing or retrosternal pain. His physical examination is normal. What would be the appropriate next investigation to perform in this patient? A. Upper Endoscopy B. Upper GI contrast study C. Esophageal manometry D. Modified Barium Swallow (MBS) E. Direct laryngoscopy 4. A 12 year old male presents to the ER after a recent episode of emesis. The parents are concerned because undigested food 3 days old was in his vomit. He admits to a sensation of food and liquids “sticking” in his chest for the past 4 months, as he points to the upper middle chest. Parents relate a 10 lb (4.5 Kg) weight loss over the past 3 months. -

Candida Ball in the Esophagus
Case Report Adv Res Gastroentero Hepatol Volume 6 Issue 1 - June 2017 DOI: 10.19080/ARGH.2017.06.555677 Copyright © All rights are reserved by Mohammad Al-Shoha Case Report: Candida Ball in the Esophagus Mohammad Al-Shoha MD1*, Nayana George MD1 and Benjamin Tharian MD1,2 1Department of Internal Medicine, University of Arkansas for Medical Sciences, USA 2Department of Medicine, Division of Gastroenterology and Hepatology, USA Submission: February 10, 2017; Published: June 16, 2017 *Corresponding author: Mohammad Al-Shoha, Department of Internal Medicine, University of Arkansas for Medical Sciences, Internal Medicine, 4301 W. Markham Street, Slo t#634, Little Rock, AR 72205, USA, Tel: , Email: [email protected] Abstract Candida albicans is the most common well known cause of infectious esophagitis. Although most patients with Candida esophagitis are immunocompromised, about 25% have scleroderma, achalasia, or other causes of esophageal dysmotility that would allow the fungi to overgrow but it has been reported in the literature that it can manifest as an esophageal mass. We are reporting a case of recurrent esophageal candidiasis manifestingand colonize on the the esophagus, Esophagogastroduodenoscopy with subsequent esophagitis (EGD) as [1,2]. an obstructing Esophageal mass. candidiasis usually manifests as white mucosal plaque-like lesions Case report showed normal complete blood count, acute renal injury with A 70-year old African American male patient who presented creatinine 11.5mg/dL, sodium 127, potassium 3.9, normal with complaints of gradually worsening dysphagia for both TSH and negative HIV testing. Repeat EGD revealed a mass at solids and liquids with an EGD revealing a nearly obstructing 25cm from incisors (image A,B) with a biopsy revealing active mass in the esophagus 25cm from incisors occupying 75-99% of esophagitis with intraepithelial fungi consistent with Candida the circumference of the esophagus with the inability to traverse with no dysplasia and malignancy. -

Nutrition Options in Short-Bowel Syndrome Upmcphysicianresources.Com/GI Instructions: Services
In This Issue 1 Nutrition Options in Short-Bowel Syndrome SPRING 2017 Division of Gastroenterology, 3 Gastric Carcinoids with Duodenal Ulcers Hepatology, and Nutrition 4 Living Donor Liver Transplant (LDLT) 6 PancreasFest 2017 / Honors and Awards 7 Pittsburgh Gut Club 8 What Is This? Nutrition Options in Short-Bowel Syndrome By David G. Binion, MD, and Zachary Zator, MD Intestinal transplantation is an option for select patients with short-bowel syndrome- associated intestinal failure (SBS-IF) who fail or do not tolerate nutritional rehabilitation. There are a range of factors to consider in the nutritional management of patients before and after intestinal transplantation. SBS-IF can be defined as the inability to maintain proper nutritional balance — including of proteins, electrolytes, macronutrients, micronutrients, and fluids — while adhering to a conventional diet in the face of an anatomically or functionally limited gut surface. The ideal management of patients with SBS-IF involves a multidisciplinary team of gastro enterologists, nurses, dietitians, pharmacists, and surgeons. Pharmacotherapeutic agents aimed at minimizing fluid losses have been routinely employed to support these patients. For instance, antidiarrheal agents, such as loperamide or diphenoxylate, are used alongside proton pump inhibitors. Somatostatin analogs, like octreotide, inhibit gastrointestinal secretions from the stomach, pancreas, and intestines and have been proven beneficial in the past. However, their role can be limited, as somatostatin can actually -

Treatment of Gastric Candidiasis in Patients with Gastric Ulcer Disease: Are Antifungal Agents Necessary?
Gut and Liver, Vol. 3, No. 1, March 2009, pp. 31-34 original article Treatment of Gastric Candidiasis in Patients with Gastric Ulcer Disease: Are Antifungal Agents Necessary? Min Kyu Jung, Seong Woo Jeon, Chang Min Cho, Won Young Tak, Young Oh Kweon, Sung Kook Kim, and Yong Hwan Choi Department of Internal Medicine, Kyungpook National University Hospital, Daegu, Korea Background/Aims: The inadequacy of information on ing; antimycotic therapy was advocated in such cases. By the treatment of gastric candidiasis with antifungal contrast, Minoli et al.3 and Gotlieb-Jensen et al.4 consid- agents promoted us to evaluate patients with fungal ered it an epiphenomenon without any significance. infections who had gastric ulcers and assess the However, because the issue remains unresolved we retro- need for proton-pump inhibitors or antifungal agents. spectively reviewed fungal infections in patients with gas- Methods: Sixteen patients were included in the study. tric ulcers to determine the need for proton pump in- The criterion for the diagnosis of candidiasis was find- hibitor and/or antifungal agents. ing yeast and hyphae in the tissue or an ulcer on histological sections of biopsy samples. Surface fungi were not considered infections. Results: In all cases MATERIALS AND METHODS with benign ulcers, follow-up endoscopy performed 6 weeks after proton-pump-inhibitor treatment revealed We reviewed the pathology specimens and medical re- that the ulcer had improved without antifungal medi- cords of patients with gastroduodenal ulcers diagnosed by cation. However, in patients with malignant ulcers, upper gastrointestinal endoscopy at Kyungpook National surgical resection was necessary for a definitive cure. -

ACCP Toolkit the 2016 ACCP Pharmacotherapy Didactic Curriculum Toolkit
ACCP Toolkit The 2016 ACCP Pharmacotherapy Didactic Curriculum Toolkit 2016 Educational Afairs Committee, American College of Clinical Pharmacy Terry L. Schwinghammer (Chair), Andrew J. Crannage (Vice Chair), Eric G. Boyce, Bridget Bradley, Alyssa Christensen, Henry M. Dunnenberger, Michelle Fravel, Holly Gurgle, Drayton A. Hammond, Jennifer Kwon, Douglas Slain, and Kurt A. Wargo. Approved by the American College of Clinical Pharmacy Board of Regents on July 18, 2016. The purpose of the 2009 and 2016 ACCP Pharmacother- ORGAN SYSTEMS AND DISEASE STATE TOPICS apy Didactic Curriculum Toolkits is to provide guid- ance to schools and colleges of pharmacy in developing, Cardiovascular Disorders maintaining, and modifying their curricula.1,2 The 2016 1 Acute coronary syndromes (STEMI, NSTEMI, unstable ACCP Educational Affairs Committee reviewed recent angina) medical literature and other documents to identify dis- 1 Atherosclerotic cardiovascular disease, primary ease states that are responsive to drug therapy. Disease prevention frequency, socioeconomic burden to society, and impact 1 Atherosclerotic cardiovascular disease, secondary of pharmacist involvement in medication therapy were prevention considered in determining which topics to include in the updated pharmacotherapy toolkit. This updated toolkit 1 Arrhythmias, atrial (e.g., atrial fbrillation) is intended to provide valuable guidance to schools and 1 Basic life support (BLS) colleges of pharmacy as they develop, maintain, and 1 Dyslipidemia modify their curricula to keep pace with major scientific 1 Heart failure, chronic advances and practice changes. 1 Hypertension In the 2016 toolkit, diseases and content topics are 1 Ischemic heart disease, stable organized by organ system, when feasible, and grouped 1 Venous thromboembolism into tiers defined by practice competency. -
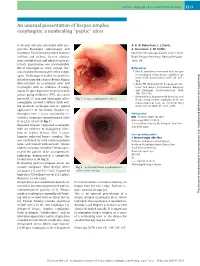
An Unusual Presentation of Herpes Simplex Esophagitis: a Nonhealing “Peptic” Ulcer
UCTN – Unusual cases and technical notes E213 An unusual presentation of herpes simplex esophagitis: a nonhealing “peptic” ulcer A 58-year-old man presented with pro- A. G. N. Robertson, L. J. Dunn, gressive dysphagia, odynophagia, and A. Immanuel, S. M. Griffin heartburn. Past history included diabetes Northern Oesophago-Gastric Cancer Unit, mellitus and asthma. Current medica- Royal Victoria Infirmary, Newcastle Upon tions included oral and inhaled corticos- Tyne, UK teroids. Examination was unremarkable. Blood investigations were normal. Bar- References ium swallow demonstrated reflux esoph- 1 Fass R. Symptom assessment tools for gas- agitis. Endoscopy revealed circumferen- troesophageal reflux disease (GERD) treat- ment. J Clin Gastroenterol 2007; 41: 437– tial ulceration and a hiatus hernia. Biopsy 444 demonstrated an esophageal ulcer and 2 Baehr PH, McDonald GB. Esophageal infec- esophagitis with no evidence of malig- tions: risk factors, presentation, diagnosis, nancy. Despite high-dose treatment with and treatment. Gastroenterology 1994; proton pump inhibitors (PPI), ulceration 106: 509 – 532 3 Ramanathan J, Rammouni M, Baran J Jr et al. persisted. CT scan and endoscopic ultra- Fig. 1 Herpes esophageal ulceration. Herpes simplex virus esophagitis in the im- sonography showed a diffuse thick-wal- munocompetent host: an overview. Am J led proximal esophagus but no typical Gastroenterol 2000; 95: 2171– 2176 appearances of carcinoma. Regular en- doscopies over 2 years consistently re- Bibliography vealed a suspicious circumferential ulcer DOI 10.1055/s-0029-1214687 from 22 to 25 cm (l" Fig. 1). Endoscopy 2009; 41: E213 Georg Thieme Verlag KG Stuttgart · New York · Repeated biopsies suggested esophagitis ISSN 0013-726X with no evidence of malignancy, infec- tion, or Crohn’s disease. -

Herpetic Esophagitis: a Diagnostic Challenge in Immunocompromised Patients
0(X)2-9270/86/8l()4-0246 THE AMERICAN JOURNAL OF GASTROENTEROLOGY Vol.81, No.4, 1986 Copyright © 1986 by Am. Coll. of Gastroenterology Primed in US.A. Herpetic Esophagitis: A Diagnostic Challenge in Immunocompromised Patients Farooq P. Agha, M.D., F.A.C.G., Horchang H. Lee, M.D., M.P.H., and Timothy T. Nostrant, M.D. Department of Radiology, and Internal Medicine-Division of Gastroenterology. University of Michigan Hospitals and Medical Center, Ann Arbor. Michigan Viral esophageal infection is eommon in immunocom- these patients to infections were; diffuse histiocytic promised patients. Twelve patients wi(b esopbagitis lymphoma in three, chronic granulocytic leukemia in secondary to herpes viruses are described. Odyno- two, diabetes mellitus in three, prolonged steroid ther- phagia, dysphagia, and gastrointestinal bleeding were apy in two, extensive burns in one, renal transplanta- the most eommon symptoms. Multiple infections par- tion in two, diffuse carcinomatosis in one, and acquired tieularly with Candida were present in three of the 12 immunedeficiency syndrome in one patient. All pa- cases (25%). Typical "volcano ulcers" at endoseopy and tients were immunosuppressed and usually multiple discrete diffusely scattered shallow uleers seen on dou- predisposing factors were responsible. All patients with ble contrast esophagram are highly suggestive of her- hematological malignancy had received extensive petic esophagitis. Single contrast esophagram plays no chemotherapy before the onset of herpetic infection. specific role in the diagnosis of herpetie esophagitis. An The pertinent clinical data on these 12 patients are analysis of elinieal, endoscopic, radiologieal, and path- summarized in Table I. ologieal features is presented. All patients were symptomatic at the time of diag- nosis. -

Predictors of Esophageal Candidiasis Among Patients Attending Endoscopy Unit in a Tertiary Hospital, Tanzania: a Retrospective Cross-Sectional Study
Predictors of esophageal candidiasis among patients attending endoscopy unit in a tertiary hospital, Tanzania: a retrospective cross-sectional study Martha F Mushi1, Nathaniel Ngeta2, Mariam M Mirambo1, Stephen E Mshana1 1. Microbiology and Immunology Department; Weill Bugando School of Medicine, P.O. Box 1464, Mwanza, Tanzania. 2. Department of Internal Medicine Weill Bugando School of Medicine. P.O. Box 1464 Mwanza, Tanzania. MFM: [email protected] NN: [email protected] MMM: [email protected] SEM: [email protected] Abstract Background: Esophageal candidiasis is a common disease among patients with impaired cell mediated immunity. In the current study, we report esophageal candidiasis among patients with various co-morbidities attending the endoscopic unit at the Bugan- do Medical Centre. Methods: This retrospective study was conducted from June to September 2015. All data of the patients who attended the endoscopic unit between 2009 and 2014 were retrieved and analyzed. Results: A total of 622 patients who underwent oesophagogastroduodenoscopy were analyzed. A slight majority 334/622(53.7%) of patients were female. Out of 622 patients; 35(5.6%) had esophageal candidiasis. Decrease in age (OR 1.1, 95%CI; 1.0-1.1), female sex (OR 3.8, 95%CI; 1.1-13.1), drinking alcohol (OR 17.1, 95%CI; 4.9-58.9), smoking (OR 8.3, 95%CI; 1.7-41.0), anti- biotic use (OR 5.7, 95%CI; 2.0-16.4), positive HIV status (OR 10.3, 95%CI; 4.6-6.0) and presence of peptic ulcer disease (OR 13.2, 95%CI; 3.5-49.0) independently predicted esophageal candidiasis. -

Herpes and Cytomegalovirus Esophagitis
E242 UCTN – Unusual cases and technical notes Herpes and cytomegalovirus esophagitis Fig. 1 Upper gastrointestinal endoscopy in a 46-year-old transplant recipient who had recently been treated with high doses of steroids showing ulcerated mucosa in: a the mid-esophagus; b,c the upper esophagus. A 46-year-old man who underwent a liver Fig. 2 Histological transplant in 2001 for fulminant hepatitis appearance of the of unknown etiology was diagnosed with esophageal ulcers a liver non-Hodgkin lymphoma (post- revealing: a herpes transplant lymphoproliferative disease) simplex virus (HSV) in 2011. Some months later, he developed inclusions within the esophageal squamous an acute hepatocellular rejection that was cells; b cytomegalovirus treated with high doses of steroids. (CMV)-infected cells by The patient was admitted because of fever immunohistochemical and severe odynophagia that was hinder- staining; c HSV-infected ing oral intake. He had multiple painful cells by immunohisto- ulcers on his tongue, palate, and oral mu- chemical staining. cosa. Upper gastrointestinal endoscopy revealed large superficial, circumferential ulcers with well-defined margins and yel- low exudate in the mid and upper esoph- agus (●" Fig.1). Biopsies taken from the ulcer base and borders confirmed herpes simplex virus (HSV) and cytomegalovirus Esophageal ulcers due to CMV are typical- A. Albuquerque1, H. Cardoso1,2, (CMV) co-infection (●" Fig. 2). Polymerase ly large, shallow, solitary or multiple, and A. Ribeiro1, E. Rios3, R. Silva3, chain reaction (PCR) of the esophageal located in the mid or distal esophagus [3]. J. Magalhães3, G. Macedo1,2 mucosa for HSV and CMV DNA was posi- In HSV esophagitis, the morphology de- 1 Gastroenterology Department, Hospital tive. -

Simultaneous Candida Albicans and Herpes Simplex Virus Type 2
Reminder of important clinical lesson BMJ Case Rep: first published as 10.1136/bcr-2019-230410 on 15 August 2019. Downloaded from Case report Simultaneous candida albicans and herpes simplex virus type 2 esophagitis in a renal transplant recipient Imran Gani, 1 Vatsalya Kosuru,2 Muhammad Saleem,2 Rajan Kapoor1 1Nephrology, Hypertension and SUMMARY episode of biopsy-proven, borderline, acute cellular Transplant Medicine, Augusta Renal transplant recipients are prone to opportunistic rejection in the second month after transplant that University Health, Augusta, infections due to iatrogenic immunosuppression. responded well to pulse steroids with normalisation Georgia, USA Infectious esophagitis can present as an opportunistic of renal function. 2Internal Medicine, Augusta infection in the post-transplant period. Common Nine months after her kidney transplantation she University Health System, Augusta, Georgia, USA pathogens are candida, herpes simplex virus (HSV) presented with sore throat, dysphagia, odynophagia and cytomegalovirus (CMV). Having a dual infection and fever. Her physical examination was significant Correspondence to is uncommon and the diagnoses can be missed at for oropharyngeal thrush, white tonsillar exudates, Dr Imran Gani, initial presentation. Our patient, a 29-year-old African- pharyngeal erythema and enlarged palatine tonsils. igani@ augusta. edu American woman, status post deceased-donor-kidney Blood work showed leukocytosis and acute kidney transplant presented with difficulty and pain in injury (AKI). She was admitted to the hospital for Accepted 22 July 2019 swallowing with clinical features suggestive of candida intravenous fluids and intravenous fluconazole esophagitis, confirmed by fungal culture. She did not therapy for severe oropharyngeal candidiasis and get better with antifungal treatment. On further testing, high suspicion of esophageal candidiasis. -

Herpes Simplex Virus and the Alimentary Tract
Herpes Simplex Virus and the Alimentary Tract Eric A. Lavery, MD , and Walter J. Coyle , MD Corresponding author infections of the gastrointestinal tract in both immuno- Walter J. Coyle, MD Division of Gastroenterology and Hepatology Scripps Clinic compromised and immunocompetent patients. Torrey Pines, 10666 North Torrey Pines Road, N203, La Jolla, CA 92037, USA. E-mail: [email protected] Background Current Gastroenterology Reports 2008, 10: 417– 423 HSV is a member of the Herpesviridae family of viruses, Current Medicine Group LLC ISSN 1522-8037 which also includes varicella zoster virus (VZV), cyto- Copyright © 2008 by Current Medicine Group LLC megalovirus (CMV), Epstein-Barr virus (EBV), and human herpesvirus (HHV) 6, 7, and 8 [ 2• ]. Members of this family contain linear, double-stranded DNA within a Herpes simplex virus (HSV) infection is well known as protein capsid, which is surrounded by a tegument and an a sexually transmitted disease. However, relatively little outer glycoprotein layer [ 2• , 3•• ]. HSV-1 and HSV-2 have has been published concerning the presentations and 70% genomic homology but tend to affect different areas treatment of HSV infection within the gastrointestinal of the body. HSV-1 tends to cause most oral and esopha- tract, where HSV most commonly affects the esophagus geal herpetic lesions; it is commonly acquired during in both immunocompromised and immunocompetent childhood, though it has been associated with proctitis in patients. HSV proctitis is not uncommon and occurs a minority of cases. HSV-1 is primarily transmitted via primarily in males having sex with males. In patients oral secretions and has a higher seroprevalence in lower with normal immune systems, gastrointestinal HSV socioeconomic communities.