Snowmelt Timing Regulates Community Composition, Phenology, and Physiological Performance of Alpine Plants
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Alplains 2013 Seed Catalog P.O
ALPLAINS 2013 SEED CATALOG P.O. BOX 489, KIOWA, CO 80117-0489, U.S.A. Three ways to contact us: FAX: (303) 621-2864 (24 HRS.) email: [email protected] website: www.alplains.com Dear Growing Friends: Welcome to our 23rd annual seed catalog! The summer of 2012 was long, hot and brutal, with drought afflicting most of the U.S. Most of my botanical explorations were restricted to Idaho, Wash- ington, Oregon and northern California but even there moisture was below average. In a year like this, seeps, swales, springs, vestigial snowbanks and localized rainstorms became much more important in my search for seeding plants. On the Snake River Plains of southern Idaho and the scab- lands of eastern Washington, early bloomers such as Viola beckwithii, V. trinervata, Ranunculus glaberrimus, Ranunculus andersonii, Fritillaria pudica and Primula cusickiana put on quite a show in mid-April but many populations could not set seed. In northern Idaho, Erythronium idahoense flowered extensively, whole meadows were covered with thousands of the creamy, pendant blossoms. One of my most satisfying finds in the Hells Canyon area had to be Sedum valens. The tiny glaucous rosettes, surround- ed by a ring of red leaves, are a succulent connoisseur’s dream. Higher up, the brilliant blue spikes of Synthyris missurica punctuated the canyon walls. In southern Oregon, the brilliant red spikes of Pedicularis densiflora lit up the Siskiyou forest floor. Further north in Oregon, large populations of Erythronium elegans, Erythronium oregonum ssp. leucandrum, Erythro- nium revolutum, trilliums and sedums provided wonderful picture-taking opportunities. Eriogonum species did well despite the drought, many of them true xerics. -

Alpine Flora
ALPINE FLORA -- PLACER GULCH Scientific and common names mostly conform to those given by John Kartesz at bonap.net/TDC FERNS & FERN ALLIES CYSTOPTERIDACEAE -- Bladder Fern Family Cystopteris fragilis Brittle Bladder Fern delicate feathery fronds hiding next to rocks and cliffs PTERIDACEAE -- Maidenhair Fern Family Cryptogramma acrostichoides American Rockbrake two different types of fronds; talus & rocky areas GYMNOSPERMS PINACEAE -- Pine Family Picea englemannii Englemann's Spruce ANGIOSPERMS -- MONOCOTS CYPERACEAE -- Sedge Family Carex haydeniana Hayden's Sedge very common alpine sedge; compact, dark, almost triangular inflorescence Eriophorum chamissonis Chamisso's Cotton-Grass Cottony head; no leaves on culm ALLIACEAE -- Onion Family Allium geyeri Geyer's Onion pinkish; onion smell LILIACEAE -- Lily Family Llyodia serotina Alp Lily white; small plant in alpine turf MELANTHIACEAE -- False Hellebore Family Anticlea elegans False Deathcamas greenish white; showy raceme above basal grass-like leaves Veratrum californicum Cornhusk Lily; CA False Hellebore greenish; huge lvs; huge plant; mostly subalpine ORCHIDACEAE -- Orchid Family Plantanthera aquilonis Green Bog Orchid greenish, in bracteate spike, spur about as long as or a bit shorter than lip POACEAE -- Grass Family Deschampsia caespitosa Tufted Hair Grass open inflorescence; thin, wiry leaves; 2 florets/spikelet; glumes longer than low floret Festuca brachyphylla ssp. coloradoensis Short-leaf Fescue dark; narrow inflorescence; thin, wiry leaves Phleum alpinum Mountain Timothy dark; -
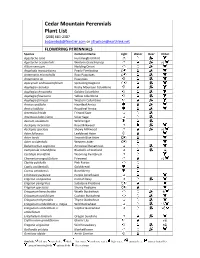
Species List
Cedar Mountain Perennials Plant List (208) 683-2387 [email protected] or [email protected] FLOWERING PERENNIALS Species Common Name Light Water Deer Other Agastache cana Hummingbird Mint Agastache occidentalis Western Giant Hyssop Allium cernuum Nodding Onion Anaphalis margaritacea Pearly Everlasting Antennaria microphylla Rosy Pussytoes Antennaria sp. Pussytoes Apocynum androsaemifolium Spreading Dogbane Aquilegia caerulea Rocky Mountain Columbine Aquilegia chrysantha Golden Columbine Aquilegia flavescens Yellow Columbine Aquilegia formosa Western Columbine Arnica cordifolia Heartleaf Arnica Arnica latifolia Broadleaf Arnica Artemisia frigida Fringed Sage Artemisia ludoviciana Silver Sage Asarum caudatum Wild Ginger Asclepias incarnata Rose Milkweed Asclepias speciosa Showy Milkweed Aster foliaceus Leafybract Aster Aster laevis Smooth Blue Aster Aster occidentalis Western Aster Balsamorhiza sagittata Arrowleaf Balsamroot Campanula rotundifolia Bluebells of Scotland Castilleja linariifolia Wyoming Paintbrush Chamerion angustifolium Fireweed Clarkia pulchella Pink Fairies Coptis occidentalis Goldthread Cornus canadensis Bunchberry Echinacea purpurea Purple Coneflower Erigeron compositus Cutleaf Daisy Erigeron peregrinus Subalpine Fleabane Erigeron speciosus Showy Fleabane Eriogonum heracleoides Wyeth Buckwheat Eriogonum ovalifolium Cushion Buckwheat Eriogonum thymoides Thyme Buckwheat Eriogonum umbellatum v. majus Wild Buckwheat Eriogonum umbellatum v. Sulphur Buckwheat umbellatum Eriophyllum lanatum Oregon Sunshine Erythronium -

Sensitive and Rare Plant Species Inventory in the Salt River and Wyoming Ranges, Bridger-Teton National Forest
Sensitive and Rare Plant Species Inventory in the Salt River and Wyoming Ranges, Bridger-Teton National Forest Prepared for Bridger-Teton National Forest P.O. Box 1888 Jackson, WY 83001 by Bonnie Heidel Wyoming Natural Diversity Database University of Wyoming Dept 3381, 1000 E. University Avenue University of Wyoming Laramie, WY 21 February 2012 Cooperative Agreement No. 07-CS-11040300-019 ABSTRACT Three sensitive and two other Wyoming species of concern were inventoried in the Wyoming and Salt River Ranges at over 20 locations. The results provided a significant set of trend data for Payson’s milkvetch (Astragalus paysonii), expanded the known distribution of Robbin’s milkvetch (Astragalus robbinsii var. minor), and relocated and expanded the local distributions of three calciphilic species at select sites as a springboard for expanded surveys. Results to date are presented with the rest of species’ information for sensitive species program reference. This report is submitted as an interim report representing the format of a final report. Tentative priorities for 2012 work include new Payson’s milkvetch surveys in major recent wildfires, and expanded Rockcress draba (Draba globosa) surveys, both intended to fill key gaps in status information that contribute to maintenance of sensitive plant resources and information on the Forest. ACKNOWLEDGEMENTS All 2011 field surveys of Payson’s milkvetch (Astragalus paysonii) were conducted by Klara Varga. These and the rest of 2011 surveys built on the 2010 work of Hollis Marriott and the earlier work of she and Walter Fertig as lead botanists of Wyoming Natural Diversity Database. This project was initially coordinated by Faith Ryan (Bridger-Teton National Forest), with the current coordination and consultation of Gary Hanvey and Tyler Johnson. -

Floristic Quality Assessment Coeffiecients of Conservatism Update
Floristic Quality Assessment Coefficients of Conservatism Update Rare Plant Symposium September 18, 2020 Pam Smith and Georgia Doyle Summary • Brief introduction to Coefficients of Conservatism and the Floristic Quality Assessment (FQA) and rationale for updating • Methods, data collection, analysis, QA/QC • Results • How you can access and use the FQA data and the taxonomic crosswalk (Ackerfield 2015, Weber & Wittmann 2001, USDA PLANTS) Floristic Quality Assessment (FQA) Managers/Landowners use to prioritize To Evaluate: land for: • Habitat conservation value • Protection • Ecological integrity • Development • Naturalness • Purchase • Restoration potential • Measure restoration effectiveness Photo: P.Smith Floristic Quality Assessment • Developed in the 1970’s for the Chicago region (Swink & Wilhelm 1979) • FQA widely used in N. America and across the world • A hybrid of judgement based assessments and quantitative metrics Floristic Quality Assessment 1) Plant list • Coefficients of Conservatism are 2) Published values numeric values (C values) assigned to each plant in the flora of a State or Region. • Values range from 0-10 and are assigned by botanical experts. Lonicera involucrata (Ackerfield 2015) Distegia involucrata (Weber & Wittmann 2001 and 2012) Lonicera involucrata var. involucrata (USDA PLANTS 2007) Lonicera involucrata (USDA PLANTS 2020) Twinberry honeysuckle C value 7 ( 5 in MT) Photo: P. Smith Coefficients of Conservatism – C values • Number assigned to an individual plant taxon is based on likelihood of finding it in an anthropogenically disturbed environment (roadside, road cut, reservoir, clear-cut, polluted, farmed, overgrazed etc.). • 0 or 1 is assigned to taxa that are almost exclusively found in human source disturbance. • A 10 is assigned to species found in a pristine habitat with a natural disturbance regime. -

December 2014 ---International Rock Gardener--- December 2014
International Rock Gardener ISSN 2053-7557 Number 60 The Scottish Rock Garden Club December 2014 ---International Rock Gardener--- December 2014 The IRG Team sends our very best wishes and thanks to all readers and contributors around the world as we come to the close of 2014. This issue of IRG is our 60th – quite a milestone for what was begun as a one year experimental project. Production of the magazine does keep the IRG Team busy but of course, none of this would be possible without the generous input of our authors and photographers: To all of you - a special greeting of warm appreciation from Margaret, ZZ, Ian, Glassford and Richard. “Don’t tell me - more last minute editorial changes” from Theresa McCracken in Oregon So far, winter in the Northern Hemisphere has been veering between cold and mild to a degree that will confuse most of our plants, while in the South a drought is expected which will lead to problems for growers there. These are, I suppose, problems which are visited upon us most years, in some way or another. Perhaps this struggle against nature’s vagaries is part of what drives us in our challenge to “conquer” the difficulties and the puzzles our gardens present to us? Or perhaps we are just all happily obsessed? Cover picture: Dryas octopetala, the plant which is the SRGC’s emblem, from a painting by Anne Chambers www.srgc.net ISSN 2053-7557 ---International Rock Gardener--- ---Plant Portrait--- A Story of Saxifraga dinnikii forma alba by Frank Schmidt, photos the author, Adrian Young and Franto Paznocht Last autumn I was asked, at the Czech-German Meeting in Feuchtwangen, to write for the IRG about the history of Saxifraga dinnikii forma alba. -

Aquilegia 39-4 Fall 2015
Newsletter of the Colorado Native Plant Society Aquilegia Workshops & Chapter Programs 2015 Photo Contest Winners Annual Conference Summary Rare Plant Symposium Report Volume 39 No. 4 Fall 2015 Biographies of Award Winners Aquilegia Volume 39, No. 4 Fall 2015 1 Second Place Photo Contest Winners The winners on each page are identified clockwise from upper left: Front Cover: First place winners (clockwise from upper left): Dave Elin (Native Plants) Penstemon grahamii, Rio Blanco County; Dave Elin (Native Plant Landscape) Populus tremuloides, San Juan Mountains; Audrey Boag (Artistic) Fern un- furling early in the morning; Lenore Mitchell (Native Plants and Wildlife) Asclepias speciosa & Bumblebee This page: Second Place Winners (clockwise from upper left): Sally Guthart (Artistic) Fallugia paradoxa, Gardens on Spring Creek, Fort Collins; Tami Kochen (Native Plant) Cirsium scopulorum, La Plata Peak; Dave Elin (Native Plant & Wildllife) Gaillardia aristata and Sweat Bee, Reynolds Park; Nicole Ellison (Native Plant Landscape) Old man of the Ute Mountain, Hymenoxys grandiflora, Ute trail RMNP 2 Aquilegia Volume 39, No. 4 Fall 2015 Colorado Native Plant Society AQUILEGIA: Newsletter of the Colorado Dedicated to furthering the knowledge, appreciation, and conservation of native plants and habitats of Colorado Native Plant Society through education, stewardship, and advocacy Inside this issue Board of Directors OFFICERS News & Announcements......................4 President(Co-) Charlie Turner [email protected] Board Meeting Summary.....................9 -

A Publication of the Wyoming Native Plant Society
Castilleja A Publication of the Wyoming Native Plant Society www.uwyo.edu/wyndd/wnps/wnps_home.htm March 2003 Volume 22 No. 1 In this issue: Megadrought 1, 4 WNPS News, Meetings 2 Phlox Family 3 Wetlands Work 5 Forest Service Region 2 Sensitive List 7 -8 Moonwort Stalking Confessions 9 - 11 Ask Linnaeus 11 Botanists Seek to Understand Megadroughts The past holds keys to the future and the clues are in the records marked by annual tree rings. University of Wyoming botanists Stephen Gray, Stephen Jackson and colleagues are publishing results of 750-year-long tree-ring chronologies examined from a network of study sites in the central and southern Rockies for multi- decade variability in precipitation (Gray et al. 2003). The study suggests that the Great Plains, the Rockies, and the Southwest are stricken by ‘megadroughts’ when the tropical Pacific turns cold at the same time that the North Atlantic warms. Parthenium alpinum Multi-year persistence of these ocean conditions a Ray floret can result in multi-year severe droughts in the b Composite flower western interior of North America. (cont. p. 4) c Leaf Illustration by B. Heidel Wyoming Harbinger of Spring Wyoming feverfew (Parthenium alpinum Smithsonian Institution on the first list of Nutt.; also called alpine feverfew) is a stemless, potentially endangered and threatened plants of mat-forming perennial forb of the Aster family the United States (Ayensu et al. 1978) but found (Asteraceae). It arises from a deep, woody to be more common and taken off the list in 1985. caudex and each branch of the caudex is crowned Currently, there are at least 44 populations known by rosettes of leaves. -

Plant Catalog and Sale Information
FREE ADMISSION MAY 11 8 a.m. – 6 p.m. MAY 12 8 a.m. – 5 p.m. PLANT CATALOG AND SALE INFORMATION IT’S YOUR TIME TO GROW! Whether you’re planting a window box, a vegetable garden or a sprawling landscape, we have plants picked just for you. Experts will be onsite to answer your questions and offer advice. Members get a 10% discount on purchases. Don’t Miss the PREVIEW PARTY MAY 10, 4-8 P.M. Enjoy delicious treats, wine and beer as you shop the greatest selection before the sale opens to the public. TICKETS $45 & LIMITED – GET YOURS TODAY! PRESENTING SPONSOR ASSOCIATE SPONSORS 10th & York Street botanicgardens.org TABLE OF CONTENTS ADMISSION & MEMBERSHIP Map 1 Entry to Spring Plant Sale is free on Friday and Saturday. Tickets are required to Annuals 2 attend the Plant Sale Preview Party on Thursday, May 10. Gardens members Aquatics 8 receive 10% off their Spring Plant Sale purchases. New this year: Buy or renew a Container Garden in a Bag 10 membership at the checkout tent when you buy your plants! Fruits, Berries and Vegetables 11 Grown at the Gardens 13 REFUND POLICY Hanging Baskets 14 All products purchased at Spring Plant Sale are non-refundable. Preview Party tickets Herbs 15 cannot be refunded or exchanged. Houseplants 17 Mixed Succulents 18 BRING YOUR WAGON! A limited number of carts will be available. We Perennial Classics 19 highly encourage guests bring their own wagons, ® Plant Select 24 wheelbarrows or carts. Rock Alpine 26 Roses 35 AMENITIES Seeds 36 • Restrooms are located in the lobby of Boettcher Memorial Center, in Marnie’s Pavilion Summer Bulbs 38 and at The Hive Garden Bistro. -

Spring 2020 Plant Sale
2020 PLANT CATALOG ONLINE SPRING Presale online shopping begins April 27 at 10 a.m. Member & volunteer online shopping is between April 28 at 10 a.m. and May 1 at 4 p.m. This catalog is not an order form. TABLE OF CONTENTS Aquatics .................................... 1 Container Garden in a Bag .......... 3 Fruits, Berries & Vegetables ........... 4 Grown at the Gardens ................. 7 Herbs ...................................... 14 Houseplants ............................. 15 Plant Select ® ............................ 16 Rock Alpine ............................. 17 Seeds ...................................... 18 Specialty Succulents .................. 20 Summer Bulbs ........................... 21 The Shop at the Gardens ........... 22 Poppy by Sue Carr, 2019, acrylics. Denver Botanic Gardens School of Botanical Art & Illustration. PRESENTING SPONSOR ASSOCIATE SPONSORS 10th & York Street botanicgardens.org AQUATICS PRICE RANGE: $5-$35 | Colorado may not be the first place that comes to mind when you think of aquatic plants, but anyone who has visited the Monet Pool in August knows they can flourish in ponds and water features in this area. You’ll find a wide variety of aquatic plants, some of which were grown on site. * Selections may vary depending on availability. American Frogbit Japanese Iris, Hall of Marble Arrowhead Japanese Iris, Melody Australia Canna Japanese Iris, Sorcerer’s Triumph Aztec Arrowhead Japanese Iris, Violet Bengal Tiger Canna Japanese Primrose Black Coral Taro Lavender Musk Blue Flag Iris Lemon Bacopa Blue -

Ethical Gardeners, Beautiful Plants by Jacqueline Heriteau
Enjoy a sunset sail on a felucca returning from the botanical gardens on Lord Horatio Herbert Kitchener Island at Aswan. one of the ports of call of the Nile Goddess on the January AHS trip to Egypt and the Nile. AHSSTUDYTOURS Awon I way to go! September 20-0ctober 3, 1990 dens in Savannah and Hilton Head. and the sig historic gardens. including the Manial Palace Gar nificant and historic gardens of Charleston . While dens in Caire and the botanical garden on Lord Castles and Gardens of Scotland cruising the Intracoastal Waterway you 'lI have op Horatio Herbert Kitchener Island at Aswan . Program In the Western Highlands of Argyll. see Culzen Park portunities to view splendid marshlands teeming leaders are Carolyn Marsh Lindsay and Bob Lind Castle and Crarae Woodland Gardens. Spend two with birds and wildlife. loin Carolyn and Bob Lind say. Mrs. Lindsay is a member of the AHS Board days at the Isle of Skye's Clan Donald Center. forty say on board this yacht cruise exploring the Colonial and the current AHS president. acres of woodland gardens and nature trails on the South . Mrs. Lindsay is the current AHS president. Leonard Haertter Travel Company grounds of Armadale Castle. Visit the highland gar Leonard Haertter Travel Company. 7922 Bonhomme Ave., dens at Inverewe before traveling on to Inverness 51. Louis. MO 63105 1800) 942-6666 lin Missouri, 314- and Edinburgh . You'll be welcomed by the castles' 721-6200). owners and guided by Everitt Miller, former director April 16-21, 1991 of Longwood Gardens and past AHS president. -

North American Rock Garden Society |
Bulletin of the American Rock Garden Society Cover: Hymenoxys grandiflora and Gentiana prostrata by Jill S. Buck of Westminister, Colorado Bulletin of the American Rock Garden Volume 51 Number 1 Winter 1993 Features Fifty Years of the ARGS Bulletin, by Mamie Flook 3 Rock Gardening in Denver—It's Different!, by Dick Bartlett 11 Growing Alpines in the Dirt, by Robert Nold 21 Growing Eriogonums in Western Washington, by Betty Lowry 25 Eriogonums: Some Botanical Notes, by Gwen Kelaidis 31 Alta, Utah: A Floral Goldmine, by David Joyner 45 A Persian Carpet Rock Garden, by Gwen Kelaidis 49 The Little Rocky Mountain Clematis, by Joan Means 55 Gardens of Vail, by Nicola Ripley 59 Departments Propagation 65 Books 67 L N. ROBERSOW |»«0 L 102* « -JUTTU 2 Bulletin of the American Rock Garden Society Vol. 51(1) Fifty Years of the ARGS Bulletin by Mamie Flook 1 he American Rock Garden Society was officially organized in New York City on March 21, 1934. Over 200 people attended a meeting planned by a group of eight rock gardening enthusiasts, including Dorothy Hansell, Florens DeBevoise, and Martha Houghton. These three ladies had met several times in 1932 and 1933 to discuss the possibility of starting a rock garden society. The background of rock gardening in the United States and the story of how our society began was thoroughly documented by Frank Cabot in his excellent essay,"As It Was in the Beginning," based on his fine presentation at the 50th Anniversary meeting of ARGS in Asheville, North Carolina in June of 1984.