Membranoproliferative Glomerulonephritis in a Young Cat
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Refractory Hypoglycemia in T-Cell Lymphoma
Open Access Austin Oncology Case Reports Case Report Refractory Hypoglycemia in T-Cell Lymphoma Buyukaydina B1*, Tunca M1, Alayb M2, Kazanciogluc R3 and Reha E3 Abstract 1Bezmialem Vakif University, Department of Internal Hypoglycemia is commonly seen in diabetes mellitus patients; whereas it Medicine, Turkey is rarely seen in a healthy person. In this case, we reported a male patient 2Yuzuncu Yil University, Department of Endocrinology, with a treatment-resistant hypoglycemia. A 53 years old male patient admitted Turkey to our clinic with debility, nausea and vomiting. Physical examination revealed 3Bezmialem Vakif University, Department of Nephology, lymphadenopathies in the left axilla and inguinal regions; and presence of right Turkey upper quadrant tenderness. Biochemical results revealed severe hypoglycemia, *Corresponding author: Banu Buyukaydin, azotemia and elevation of liver enzymes. Histological result of the excisional Bezmialem Vakif University, Department of Internal lymph node biopsy was compatible with peripheral T cell lymphoma. In ward, Medicine, Turkey the patient has repeated recurrent hypoglycemia, which did not resolve with all treatment given. His general condition deteriorated and he died due to sepsis. Received: June 01, 2016; Accepted: July 10, 2016; This case highlighted the need to rule out hematologic malignancies; precisely Published: July 13, 2016 T-cell lymphoma in a patient who presented with resistant hypoglycemia in the presence of lymphadenopathy. Keywords: Hypoglycemia, Lymphoma, IGF-II Introduction approximately fifty percent of proliferation index. CD3 was positive. This finding was compatible to histological diagnosis of peripheral Hypoglycemia is defined as the occurrence of a variety of T-cell lymphoma with partial involvement of lymph ganglia. symptoms in association with plasma glucose concentration of 50mg/dl or less. -

Clinical and Histopathological Features of Renal Maldevelopment in Boxer Dogs: a Retrospective Case Series (1999–2018) †
animals Article Clinical and Histopathological Features of Renal Maldevelopment in Boxer Dogs: A Retrospective Case Series (1999–2018) † Maria Alfonsa Cavalera 1, Floriana Gernone 1, Annamaria Uva 1, Paola D’Ippolito 2, Xavier Roura 3 and Andrea Zatelli 1,* 1 Department of Veterinary Medicine, University of Bari, 70010 Valenzano, Italy; [email protected] (M.A.C.); fl[email protected] (F.G.); [email protected] (A.U.) 2 Veterinary diagnostic Lab ACV Triggiano, 70019 Triggiano, Italy; [email protected] 3 Hospital Clínic Veterinari, Universitat Autònoma de Barcelona, 08193 Bellaterra, Spain; [email protected] * Correspondence: [email protected]; Tel.: +39-080-4679804 † This study was partially presented as oral communication at the 11th ECVIM-CA/ESVIM Congress, Dublin (Ireland) as “Congenital nephrotic syndrome with renal glomerular immaturity in 7 Boxer dogs”. Zatelli, A., Domenech, O., Bussadori, C., Lubas, G., Del Piero, F. Simple Summary: This study describes clinical findings in Boxer dogs with renal maldevelopment and proposes a possible mode of inheritance. Medical records of 9 female Boxer dogs, older than 5 months and with a clinical diagnosis of proteinuric chronic kidney disease prior to one year of age, showed the presence of polyuria and polydipsia, decreased appetite, weight loss, lethargy and weakness in all affected dogs. Common laboratory findings were proteinuria and diluted urine, non- regenerative anemia, azotemia, hyperphosphatemia, hypoalbuminemia and hypercholesterolemia. Citation: Cavalera, M.A.; Gernone, Histopathology of the kidneys identified the presence of immature glomeruli in all dogs. In 7 out F.; Uva, A.; D’Ippolito, P.; Roura, X.; of 9 related dogs, the pedigree analysis showed that a simple autosomal recessive trait may be a Zatelli, A. -

SIRS Is Valid in Discriminating Between Severe and Moderate Diabetic Foot Infections
Pathophysiology/Complications ORIGINAL ARTICLE SIRS Is Valid in Discriminating Between Severe and Moderate Diabetic Foot Infections 1 2 DANE K. WUKICH, MD KATHERINE MARIE RASPOVIC, DPM best of our knowledge, the use of SIRS 2 3 KIMBERLEE B. HOBIZAL, DPM BEDDA L. ROSARIO, PHD has not yet been validated as a method of discriminating between moderate and severe DFI. OBJECTIVEdThis retrospective, single-center study was designed to distinguish severe di- The aim of this study was to classify abetic foot infection (DFI) from moderate DFI based on the presence or absence of systemic fl infectionseverityinagroupofhospital- in ammatory response syndrome (SIRS). ized diabetic patients based on the pres- RESEARCH DESIGN AND METHODSdThe database of a single academic foot and ence or absence of SIRS. The reason for ankle program was reviewed and 119 patients were identified. Severe DFI was defined as local hospitalization in this group of patients infection associated with manifestation of two or more objective findings of systemic toxicity was their DFI. Our hypotheses are that using SIRS criteria. patients with DFI who manifest SIRS (i.e., severe infection) will have longer hospital RESULTSdPatients with severe DFI experienced a 2.55-fold higher risk of any amputation – – stays and higher rates of major amputa- (95% CI 1.21 5.36) and a 7.12-fold higher risk of major amputation (1.83 41.05) than patients tion than patients who don’tmanifest with moderate DFI. The risk of minor amputations was not significantly different between the two groups (odds ratio 1.02 [95% CI 0.51–2.28]). The odds of having a severe DFI was 7.82 SIRS (i.e., moderate infection). -

Drug-Induced Hyperglycemia Risk Factors Presentation Causative Agents Mallory Linck, Pharm.D
9/29/12 Outline Drug-Induced Hyperglycemia Risk Factors Presentation Causative Agents Mallory Linck, Pharm.D. Mechanisms Pharmacy Practice Resident Prevention University of Arkansas for Medical Sciences Management Risk Factors Presentation of Drug-Induced Hyperglycemia Mild-to-Moderate Severe disease General Diabetes risk factors, plus: Blurred vision Abdominal Pain, N/V Pre-existing or underlying Diabetes Excessive thirst Coma Higher doses of thiazides or corticosteroids Fatigue/weakness Dehydration Use of more than one drug that can induce Polydipsia Hypokalemia hyperglycemia Polyphagia Hypotension Polypharmacy Polyuria Kussmaul respiration and Unexplained weight loss fruity breath Increased Blood Glucose Lethargy Metabolic acidosis Causative Agents Mechanisms Atypical antipsychotics Nicotinic acid B-blockers Oral contraceptives Cyclosporine Pentamidine Diazoxide Phenothiazines Thiazide Diuretics Phenytoin Fish oil Protease inhibitors Glucocorticoids Rifampin Growth Hormone Ritodrine Interferons Tacrolimus Megesterol Terbutaline Thalidomide 1 9/29/12 Thiazide Diuretics High doses Hypokalemia “Each 0.5mEq/L decrease in serum potassium was associated with a 45% higher risk of new diabetes” DECREASE INSULIN SECRETION Plan: Use smaller doses (12.5-25mg/day HCTZ) and replace potassium Shafi T. Hypertension. 2009 Feb;53(2):e19. Immunosuppressants Overall incidence 4-46% Pre-disposing factors Genetics Metabolic syndrome Increasing age Most common in the first few months post- transplant -

Understanding Your Pet's Blood Work…
Understanding your pet’s blood work… TO HELP YOU UNDERSTAND YOUR PET’s TEST RESULTS, THIS GUIDE EXPLAINS COMMON TESTS AND INDICES PERTINENT TO ANIMAL HEALTH. COMPLETE BLOOD COUNT (CBC) A CBC gives information on hydration status, anemia, infection, the blood’s clotting ability, and the ability of the immune system to respond. This test is essential for pets with fevers, vomiting, diarrhea, weakness, pale gums, or loss of appetite. If you pet needs surgery, a CBC can detect bleeding disorders or other unseen abnormalities. o HCT (Hematocrit) - measures the percentage of red o Lymphocytes/ Monocytes/ Neutrophils - specific blood cells to detect anemia and dehydration. types of WBCs o Hb or MCHC (Hemoglobin and mean corpuscular o Eosinophils - specific type of WBC that may indicate hemoglobin concentration) - help determine the allergic or parasitic conditions. blood’s ability to carry oxygen. o PTL (Platelet count) - type of blood element that is o WBC (White blood cell count) - measures the body’s involved in the clotting process. immune cells. Increases or decreases indicate certain disease or infections. BLOOD CHEMISTRIES These common blood serum tests help evaluate organ function, electrolyte status, hormone levels, and more. They are important in evaluating older pets, pets with vomiting and diarrhea or toxin exposure, pets receiving long-term medications, and health before anesthesia. o ALB (Albumin)-is serum protein that helps evaluate o GLOB (Globulin) - is a blood protein that can be hydration, hemorrhage, and intestinal, liver, and abnormal with chronic inflammation and certain kidney disease. gastrointestinal disorders. o ALKP (Alkaline Phosphatase) - elevations may o GLU (Glucose) – is the blood sugar level. -

Azotemia & Acute Kidney Injury
AZOTEMIA & ACUTE KIDNEY INJURY Gregory F. Grauer, DVM, MS, DACVIM (SAIM) Sarah Guess, DVM, MS Kansas State University FIRST-TIME DIAGNOSIS OF AZOTEMIA OR ACUTE-ONSET AZOTEMIA INVESTIGATION Assess USG HYPERSTHENURIA PRESENT NO Rule out postrenal azotemia (dogs, ≥1.030; cats, ≥1.035)? (from obstruction or leakage) with ultrasonography and positive contrast imaging, if necessary YES TREATMENT Probable renal azotemia TREATMENT Reassess adequacy of If prerenal dehydration/azotemia confirmed volume replacement (eg, skin tenting, tacky mucous membranes, after 24 and 48 hours; elevated serum albumin concentration), initiate continue fluid therapy fluid therapy to rehydrate patient (Table 2) INVESTIGATION Rule out CKD and acute-on-chronic kidney disease (Table 1) Azotemia resolves? NO YES TREATMENT Initiate fluid therapy (if not already started) to rehydrate patient Volume-responsive azotemia (Table 2) DIAGNOSIS Prerenal azotemia superimposed on YES Azotemia resolves? inability to concentrate urine (eg, diabetes mellitus, hypoadrenocorticism, hypercalcemia, pyometra) NO Suspect AKI and pursue AKI = acute kidney injury therapy (page 30) CKD = chronic kidney disease USG = urine specific gravity 28 cliniciansbrief.com October 2016 MANAGEMENT TREE h NEPHROLOGY h PEER REVIEWED TABLE 1 TABLE 2 HISTORY, EXAMINATION, EXAMPLE OF FLUID VOLUME & LABORATORY FINDINGS REQUIREMENTS FOR A 20-KG DOG OF CKD & AKI* WITH 8% DEHYDRATION & AKI Findings CKD AKI Deficit needs (8% × 20 kg) = 1600 mL over 6 hours (replace over 4-6 hours) Weight loss, poor coat, poor body -

Rhabdomyolysis in Intensive Care Unit: More Than One Cause Puneet Saxena1, Sahajal Dhooria2, Ritesh Agarwal3, Kuruswamy Thurai Prasad4, Inderpaul Singh Sehgal5
CASE REPORT Rhabdomyolysis in Intensive Care Unit: More than One Cause Puneet Saxena1, Sahajal Dhooria2, Ritesh Agarwal3, Kuruswamy Thurai Prasad4, Inderpaul Singh Sehgal5 ABSTRACT Rhabdomyolysis is a serious medical condition, encountered in the intensive care unit (ICU). The etiology of rhabdomyolysis is often multifactorial. It leads to complications like acute kidney injury and life-threatening electrolyte abnormalities. A high index of suspicion and early institution of therapy is required to prevent complications and improve patient outcomes. Herein, we present the case of a young man with alcohol dependence who presented with fever and altered sensorium. He was found to have rhabdomyolysis and was managed successfully. We also discuss the common causes of rhabdomyolysis and a bedside approach to its management in the ICU. Keywords: Delirium tremens, Renal failure, Rhabdomyolysis, Tropical myositis Indian Journal of Critical Care Medicine (2019): 10.5005/jp-journals-10071-23238 INTRODUCTION 1-5Department of Pulmonary Medicine, Postgraduate Institute of Rhabdomyolysis is a condition characterized by breakdown of Medical Education and Research, Chandigarh, India skeletal muscles. This leads to the release of toxic intracellular Corresponding Author: Inderpaul Singh Sehgal, Department of contents into the systemic circulation.1 It is diagnosed by the Pulmonary Medicine, Postgraduate Institute of Medical Education presence of significantly elevated levels of creatine kinase (CK), and Research, Chandigarh, India, Phone: 91-172-2746823, e-mail: and the excretion of myoglobin in the urine (myoglobinuria), [email protected] which may impart a cola color to the urine.1 Rhabdomyolysis was How to cite this article: Saxena P, Dhooria S, Agarwal R, Prasad KT, initially described in the victims of war and natural disasters with Sehgal IS. -

Understanding Your Pet's Blood Work
Understanding your pet’s blood work Blood tests help us determine your pet’s health status and causes of illness accurately, safely, and quickly let us monitor the progress of medical treatments. A checkmark in any box below indicates a significant abnormality on your pet’s blood work. On the back of this sheet are remarks from the doctor with more details on your pet’s blood work results. Complete Blood Count (CBC) WBC (white blood cell) count classifies and The most common test, a CBC, gives information measures the body’s immune cells. Increases or on hydration status, anemia, infection, the decreases indicate certain diseases or infections. blood’s clotting ability, and the immune system’s ability to respond. EOS (eosinophils) are a specific type of white blood cells that, if elevated, may indicate allergic HCT (Hematocrit) measures the percentage of or parasitic conditions. red blood cells to detect anemia and dehydration. PLT (platelet count) measures cells that help Hb and MCHC (hemoglobin and mean stop bleeding by forming clots. corpuscular hemoglobin concentration) measures hemoglobin, the oxygen-carrying pigment of red RETICS (reticulocytes) are immature red blood blood cells (corpuscles). cells. High or low levels help classify anemias. GRANS and L/M (granulocytes and lymphocytes/ monocytes) are specific types of white blood cells. Serum Chemistry Profile ALT (alanine aminotrasferase) is PHOS (phosphorus) elevations a sensitive indicator of active liver are often associated with kidney These common tests evaluate damage but doesn’t indicate the disease, hyperthyroidism, and organ function, electrolyte status, cause. bleeding disorders. hormone levels, and more. Comprehensive Profile Ca (calcium) deviations can in- Wellness Profile dicate a variety of diseases. -

The Meaning of the Blood Urea Nitrogen/Creatinine Ratio in Acute Kidney Injury
Clin Kidney J (2012) 5: 187–191 doi: 10.1093/ckj/sfs013 Personal Opinion The meaning of the blood urea nitrogen/creatinine ratio in acute kidney injury Shigehiko Uchino1, Rinaldo Bellomo2,3 and Donna Goldsmith2,3 1Intensive Care Unit, Department of Anesthesiology, Jikei University school of Medicine, Tokyo, Japan, 2Department of Intensive Care, Austin Hospital, Melbourne, Australia and 3Department of Medicine, Austin Hospital, Melbourne, Australia Correspondence and offprint requests to: Rinaldo Bellomo; E-mail: [email protected] Abstract Background. A blood urea nitrogen (BUN)/creatinine ratio (BCR) >20 (0.081 in international unit) is used to distinguish pre-renal azotemia (PRA) and acute tubular necrosis (ATN). However, there is little evidence that BCR can distinguish between these two conditions and/or is clinically useful. Methods. We conducted a retrospective study using a large hospital database. Patients were divided into three groups: ‘low BCR’ (if BCR when acute kidney injury (AKI) developed was 20), ‘high BCR’ (if BCR when AKI developed was >20) and ‘no AKI’ if patients did not satisfy any of the Risk, Injury, Failure, Loss and End-stage kidney disease criteria for AKI during hospitalization. Results. Among 20 126 study patients, 3641 (18.1%) had AKI. Among these patients, 1704 (46.8%) had a BCR <20 at AKI diagnosis (‘low BCR’) and 1937 (53.2%) had a BCR >20 (‘high BCR’). The average BCR for the two groups was 15.8 versus 26.1 (P < 0.001). Hospital mortality was significantly less in the ‘low-BCR’ group (18.4 versus 29.9%, P < 0.001). Multivariable logistic regression analysis for hospital mortality (‘no AKI’ as a reference) showed that the odds ratio of ‘high BCR’ (5.73) was higher than that of ‘low BCR’ (3.32). -
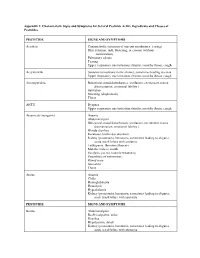
Appendix 2: Characteristic Signs and Symptoms for Several Pesticide Active Ingredients and Classes of Pesticides
Appendix 2: Characteristic Signs and Symptoms for Several Pesticide Active Ingredients and Classes of Pesticides PESTICIDE SIGNS AND SYMPTOMS Acrolein Conjunctivitis (irritation of mucous membranes, tearing) Skin irritation, rash, blistering, or erosion (without sensitization) Pulmonary edema Tearing Upper respiratory tract irritation: rhinitis, scratchy throat, cough Acrylonitrile Seizures/convulsions (tonic-clonic), sometimes leading to coma Upper respiratory tract irritation: rhinitis, scratchy throat, cough Aminopyridine Behavioral-mood disturbances (confusion, excitement, mania, disorientation, emotional lability ) Salivation Sweating (diaphoresis) Thirst ANTU Dyspnea Upper respiratory tract irritation: rhinitis, scratchy throat, cough Arsenicals (inorganic) Anemia Abdominal pain Behavioral-mood disturbances (confusion, excitement, mania, disorientation, emotional lability ) Bloody diarrhea Keratoses, brown discoloration Kidney (proteinuria, hematuria, sometimes leading to oliguria, acute renal failure with azotemia Leukopenia, thrombocytopenia Metallic taste in mouth Paralysis, paresis (muscle weakness) Paresthesia of extremities Runny nose Stomatitis Thirst Arsine Anemia Chills Hemoglobinuria Hemolysis Hyperkalemia Kidney (proteinuria, hematuria, sometimes leading to oliguria, acute renal failure with azotemia PESTICIDE SIGNS AND SYMPTOMS Borate Abdominal pain Beefy red palms, soles Diarrhea Hypotension, shock Kidney (proteinuria, hematuria, sometimes leading to oliguria, acute renal failure with azotemia Nervous system depression -

Albumin Interpretive Summary
Albumin Interpretive Summary Description: Albumin is an important regulator of osmotic equilibrium in the body and is also a carrier for highly protein-bound substances (e.g. calcium, thyroxine, fatty acids, and some drugs). Decreased Albumin Common Causes Decreased production o Liver disease . Atrophy . Fibrosis/cirrhosis . Portosystemic shunt . Neoplasia o Maldigestion . Exocrine pancreatic insufficiency (EPI) o Malabsorption . Small intestinal disease o Malnutrition . Cachexia . Dietary deficiency . Parasites o Inflammation (negative acute phase reactant) o Compensatory (with hyperglobulinemia) Increased loss o Hemorrhage (especially external) . Gastrointestinal [GI] tract . Urinary tract . Other o Protein-losing nephropathy (PLN) . Glomerulonephritis . Amyloidosis o Protein-losing enteropathy (PLE) . Lymphangiectasia . Inflammatory bowel disease [IBD] . Neoplasia . Fungal infection . Intestinal parasitism o Addison’s disease Hemodilution o Excess administration of intravenous fluid Uncommon Causes Decreased production o Neonates o Pregnancy, lactation o Maldigestion/malabsorption . Brush border enzyme deficiency Increased loss o Protein-losing dermatopathy Generated by VetConnect® PLUS: Albumin Page 1 of 3 . Burns . Severe exudative skin disease . Vasculitis . Trauma o High-protein effusions . Pancreatitis . Peritonitis . Vasculitis Hemodilution o Edema disorders . Congestive heart failure . Nephrotic syndrome . Hydrothorax . Ascites o Concurrent hypovolemia and increased total body water . Fluid accumulation in a third space -

Understanding Your Pet's Blood Work
Understanding your pet’s blood work Blood tests help us determine your pet’s health status and causes of illness accurately, safely, and quickly and let us monitor the progress of medical treatments. A checkmark in any box indicates a significant abnormal finding on your pet’s blood work. If you have questions, ask any staff member. We want you to understand our recommendations and be a partner in your pet’s care. Complete blood count (CBC) The most common test, a CBC gives information on > WBC (white blood cell) count classifies and hydration status, anemia, infection, the blood’s clotting measures the body’s immune cells. Increases or ability, and the immune system’s ability to respond. decreases indicate certain diseases or infections. > HCT (hematocrit) measures the percentage of red > EOS (eosinophils) are a specific type blood cells to detect anemia and dehydration. of white blood cells that, if elevated, may indicate allergic or parasitic conditions. > Hb and MCHC (hemoglobin and mean corpuscular hemoglobin concentration) measure hemoglobin, the > PLT (platelet count) measures cells that help oxygen-carrying pigment of red blood cells (corpuscles). stop bleeding by forming blood clots. > GRANS and L/M (granulocytes and lymphocytes/ > RETICS (reticulocytes) are immature red blood monocytes) are specific types of white blood cells cells. high or low levels help classify anemias. Serum chemistry profile ese common tests evaluate > CHOL (cholesterol) is used levels may indicate kidney failure, organ function, electrolyte status, to supplement diagnosis of Addison’s disease, dehydration, and hypothyroidism, liver disease, urethral obstruction. High levels can hormone levels, and more.