Application of Isotope Techniques to Xa9848334 Investigate Groundwater Pollution in India
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Annual Report 2018-2019
ANNUAL REPORT 2018-2019 STATE POLLUTION CONTROL BOARD, ODISHA A/118, Nilakantha Nagar, Unit-Viii Bhubaneswar SPCB, Odisha (350 Copies) Published By: State Pollution Control Board, Odisha Bhubaneswar – 751012 Printed By: Semaphore Technologies Private Limited 3, Gokul Baral Street, 1st Floor Kolkata-700012, Ph. No.- +91 9836873211 Highlights of Activities Chapter-I 01 Introduction Chapter-II 05 Constitution of the State Board Chapter-III 07 Constitution of Committees Chapter-IV 12 Board Meeting Chapter-V 13 Activities Chapter-VI 136 Legal Matters Chapter-VII 137 Finance and Accounts Chapter-VIII 139 Other Important Activities Annexures - 170 (I) Organisational Chart (II) Rate Chart for Sampling & Analysis of 171 Env. Samples 181 (III) Staff Strength CONTENTS Annual Report 2018-19 Highlights of Activities of the State Pollution Control Board, Odisha he State Pollution Control Board (SPCB), Odisha was constituted in July, 1983 and was entrusted with the responsibility of implementing the Environmental Acts, particularly the TWater (Prevention and Control of Pollution) Act, 1974, the Water (Prevention and Control of Pollution) Cess Act, 1977, the Air (Prevention and Control of Pollution) Act, 1981 and the Environment (Protection) Act, 1986. Several Rules addressing specific environmental problems like Hazardous Waste Management, Bio-Medical Waste Management, Solid Waste Management, E-Waste Management, Plastic Waste Management, Construction & Demolition Waste Management, Environmental Impact Assessment etc. have been brought out under the Environment (Protection) Act. The SPCB also executes and ensures proper implementation of the environmental policies of the Union and the State Government. The activities of the SPCB broadly cover the following: Planning comprehensive programs towards prevention, control or abatement of pollution and enforcing the environmental laws. -

Organic Matter Depositional Microenvironment in Deltaic Channel Deposits of Mahanadi River, Andhra Pradesh
AL SC R IEN 180 TU C A E N F D O N U A N D D A E I T Journal of Applied and Natural Science 1(2): 180-190 (2009) L I O P N P JANS A ANSF 2008 Organic matter depositional microenvironment in deltaic channel deposits of Mahanadi river, Andhra Pradesh Anjum Farooqui*, T. Karuna Karudu1, D. Rajasekhara Reddy1 and Ravi Mishra2 Birbal Sahni Institute of Palaeobotany, 53, University Road, Lucknow, INDIA 1Delta Studies Institute, Andhra University, Sivajipalem, Visakhapatnam-17, INDIA 2ONGC, 9, Kaulagarh Road, Dehra dun, INDIA *Corresponding author. E-mail: [email protected] Abstract: Quantitative and qualitative variations in microscopic plant organic matter assemblages and its preservation state in deltaic channel deposits of Mahanadi River was correlated with the depositional environment in the ecosystem in order to prepare a modern analogue for use in palaeoenvironment studies. For this, palynological and palynofacies study was carried out in 57 surface sediment samples from Birupa river System, Kathjodi-Debi River system and Kuakhai River System constituting Upper, Middle and Lower Deltaic part of Mahanadi river. The apex of the delta shows dominance of Spirogyra algae indicating high nutrient, low energy shallow ecosystem during most of the year and recharged only during monsoons. The depositional environment is anoxic to dysoxic in the central and south-eastern part of the Middle Deltaic Plain (MDP) and Lower Deltaic Plain (LDP) indicated by high percentage of nearby palynomorphs, Particulate Organic Matter (POM) and algal or fungal spores. The northern part of the delta show high POM preservation only in the estuarine area in LDP but high Amorphous Organic Matter (MOA) in MDP. -

Rise and Fall of Buddhism on Daya Basin
Orissa Review * December - 2007 Rise and Fall of Buddhism on Daya Basin Dr. Saroj Kumar Panda River Daya which originates from the river teachers used to impart here both religious and Kuakhai at Balakati near Hirapur (famous for secular instructions to people. These teachers Chausathi Yogini temple) has a south western were greatly loved and respected by the simple course of about 45 miles. It flows through Uttara, country folk for the blessed hopes they gave to Dhauli, Kakudia, Aragarh, Beguniapara, their afflicted hearts. In course of time some of Pandiakera, Balabhadrapur and finally discharges these monasteries grew up into famous university into Chilika lake.1 On its course, Daya is joined centres. As torch bearer of the Buddhist culture by the Bhargavi river, the Gangua Nalla, the these centres attracted pupils and scholars from Malaguni river, the Luna river and many smaller far and wide.3 drainages from Khurda sub-division.2 Two The development of Mahayan Buddhism important Buddhist vestige, whose traces are in Orissa may be studied through the historical found today on the Daya basin is highlighted in growth of these monastic institutions and through this paper. the activities of the sages and philosophers of this Buddhism in Orissa flourished during the religion. The Nagarjuni Konda inscription early Christian era independent of the Kusan engraved during 14th year of the Mahariputa patronage. In fact, till the coming of the Bhaumakar Virapurusadatta, testifies to the development of dynasty in the 8th century A.D., notable Buddhist some Hinayanic strongholds at Tosali, Palura, rulers were not known to have thrived here more Hirumu, Papila and Puspagiri by 3rd century A.D. -

The Life of Krishna Chaitanya
The Life of Krishna Chaitanya first volume of the series: The Life and Teachings of Krishna Chaitanya by Parama Karuna Devi published by Jagannatha Vallabha Vedic Research Center (second edition) Copyright © 2016 Jagannatha Vallabha Vedic Research Center All rights reserved. ISBN-13: 978-1532745232 ISBN-10: 1532745230 Our Jagannatha Vallabha Vedic Research Center is a non-profit organization, dedicated to the research, preservation and propagation of Vedic knowledge and tradition, commonly described as “Hinduism”. Our main work consists in publishing and popularizing, translating and commenting the original scriptures and also texts dealing with history, culture and the peoblems to be tackled to re-establish a correct vision of the original Tradition, overcoming sectarianism and partisan political interests. Anyone who wants to cooperate with the Center is welcome. We also offer technical assistance to authors who wish to publish their own works through the Center or independently. For further information please contact: Mataji Parama Karuna Devi [email protected], [email protected] +91 94373 00906 Contents Introduction 11 Chaitanya's forefathers 15 Early period in Navadvipa 19 Nimai Pandita becomes a famous scholar 23 The meeting with Keshava Kashmiri 27 Haridasa arrives in Navadvipa 30 The journey to Gaya 35 Nimai's transformation in divine love 38 The arrival of Nityananda 43 Advaita Acharya endorses Nimai's mission 47 The meaning of Krishna Consciousness 51 The beginning of the Sankirtana movement 54 Nityananda goes begging -
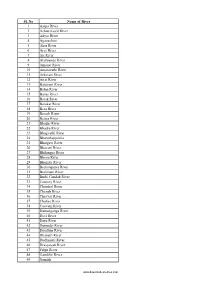
List of Rivers in India
Sl. No Name of River 1 Aarpa River 2 Achan Kovil River 3 Adyar River 4 Aganashini 5 Ahar River 6 Ajay River 7 Aji River 8 Alaknanda River 9 Amanat River 10 Amaravathi River 11 Arkavati River 12 Atrai River 13 Baitarani River 14 Balan River 15 Banas River 16 Barak River 17 Barakar River 18 Beas River 19 Berach River 20 Betwa River 21 Bhadar River 22 Bhadra River 23 Bhagirathi River 24 Bharathappuzha 25 Bhargavi River 26 Bhavani River 27 Bhilangna River 28 Bhima River 29 Bhugdoi River 30 Brahmaputra River 31 Brahmani River 32 Burhi Gandak River 33 Cauvery River 34 Chambal River 35 Chenab River 36 Cheyyar River 37 Chaliya River 38 Coovum River 39 Damanganga River 40 Devi River 41 Daya River 42 Damodar River 43 Doodhna River 44 Dhansiri River 45 Dudhimati River 46 Dravyavati River 47 Falgu River 48 Gambhir River 49 Gandak www.downloadexcelfiles.com 50 Ganges River 51 Ganges River 52 Gayathripuzha 53 Ghaggar River 54 Ghaghara River 55 Ghataprabha 56 Girija River 57 Girna River 58 Godavari River 59 Gomti River 60 Gunjavni River 61 Halali River 62 Hoogli River 63 Hindon River 64 gursuti river 65 IB River 66 Indus River 67 Indravati River 68 Indrayani River 69 Jaldhaka 70 Jhelum River 71 Jayamangali River 72 Jambhira River 73 Kabini River 74 Kadalundi River 75 Kaagini River 76 Kali River- Gujarat 77 Kali River- Karnataka 78 Kali River- Uttarakhand 79 Kali River- Uttar Pradesh 80 Kali Sindh River 81 Kaliasote River 82 Karmanasha 83 Karban River 84 Kallada River 85 Kallayi River 86 Kalpathipuzha 87 Kameng River 88 Kanhan River 89 Kamla River 90 -

GI Journal No. 51 1 September 30, 2013
GI Journal No. 51 1 September 30, 2013 GOVERNMENT OF INDIA GEOGRAPHICAL INDICATIONS JOURNAL NO. 51 SEPTEMBER 30, 2013 / ASVINA 08, SAKA 1935 GI Journal No. 51 2 September 30, 2013 INDEX S.No. Particulars Page No. 1. Official Notices 4 2. New G.I Application Details 5 3. Public Notice 7 4. GI Applications Orissa Pattachitra (Logo) – GI Application No. 386 8 Bastar Dhokra (Logo) – GI Application No. 387 16 Bell Metal ware of Datia and Tikamgargh (Logo) – GI Application No. 388 25 5. General Information 32 6. Registration Process 34 GI Journal No. 51 3 September 30, 2013 OFFICIAL NOTICES Sub: Notice is given under Rule 41(1) of Geographical Indications of Goods (Registration & Protection) Rules, 2002. 1. As per the requirement of Rule 41(1) it is informed that the issue of Journal 51 of the Geographical Indications Journal dated 30th September 2013 / Asvina 08th, Saka 1935 has been made available to the public from 30th September 2013. GI Journal No. 51 4 September 30, 2013 NEW G.I APPLICATION DETAILS App.No. Geographical Indications Class Goods 405 Makrana Marble 19 Natural Goods 406 Salem Mango 31 Horticulture 407 Hosur Rose 31 Agricultural 408 Payyanur Pavithra Mothiram 14 Handicraft 409 Kodali Karuppur Saree 24 & 25 Textile 410 Thammampatti Wood Carvings 20 Handicraft 411 Rajapalayam Lock 6 Manufactured 412 Chamba Painting 16 Handicraft 413 Kangra Paintings 16 Handicraft 414 Punjabi Jutti 25 Handicraft 415 Aipan 16 Handicraft 416 Lahaul & Spiti Wool Weaving 23 Handicraft 417 Lacquer Ware Furniture 20 Handicraft 418 Jhajjar Pottery 21 Handicraft 419 Tamta Copperware Craft 6 Handicraft 420 Rewari Jutti 25 Handicraft 421 Hoshiarpur Wood Inlay 20 Handicraft 422 Kandangi Sarees 24 Textile 423 Thanjavur Pith Works 20 Handicraft 424 Karupur Kalamkari Paintings 24 Handicraft 425 Thanjavur Cut Glass Work 20 Handicraft 426 Mahabalipuram Stone Sculpture 19 Handicraft 427 Nagercoil Temple Car 20 Handicraft 428 Kannyakumari Stone Carving 19 Handicraft 429 Arumbavur Wood Carving 20 Handicraft GI Journal No. -

Puri District, Odisha
कᴂ द्रीय भूमि जल बो셍ड जल संसाधन, नदी विकास और गंगा संरक्षण विभाग, जल शक्ति मंत्रालय भारि सरकार Central Ground Water Board Department of Water Resources, River Development and Ganga Rejuvenation, Ministry of Jal Shakti Government of India AQUIFER MAPPING AND MANAGEMENT OF GROUND WATER RESOURCES PURI DISTRICT, ODISHA दक्षक्षण पूिी क्षेत्र, भुिने�िर South Eastern Region, Bhubaneswar Government of India Ministry of Jal Shakti DEPARTMENT OF WATER RESOURCES, RIVER DEVELOPMENT & GANGA REJUVENATION AQUIFER MAPPING AND MANAGEMENT PLAN OF PURI DISTRICT ODISHA BY CHIRASHREE MOHANTY (SCIENTIST-C) CENTRAL GROUND WATER BOARD South Eastern Region, Bhubaneswar July – 2018 HYDROGEOLOGICAL FRAMEWORK, GROUND WATER DEVELOPMENT PROSPECTS & AQUIFER MANAGEMENT PLAN IN PARTS OF PURI DISTRICT, ODISHA CONTRIBUTORS PAGE Data Acquisition : Sh. D. Biswas, Scientist-‘D’ Sh Gulab Prasad, Scientist-‘D’ Smt. C. Mohanty, Scientist-‘C’ Shri D. N.Mandal, Scientist-‘D’ Shri S.Sahu- Scientist-‘C’ Data Processing : Smt. C. Mohanty, Scientist-‘C’ Shri D. Biswas, Scientist-‘D’ Dr. N. C. Nayak, Scientist-‘D’ Data Compilation & Editing: Smt. C. Mohanty, Scientist-‘C’ Dr. N. C. Nayak, Scientist-‘D’ Data Interpretation : Smt. C. Mohanty, Scientist-‘C’ Dr. N. C. Nayak, Scientist-‘D’ GIS : Smt. C. Mohanty, Scientist-‘C’ Dr. N. C. Nayak, Scientist-‘D’ Shri P. K. Mohapatra, Scientist-‘D’ Report Compilation : Smt. C. Mohanty, Scientist-‘C’ Technical Guidance : Shri P. K. Mohapatra, Scientist-‘D’ Shri S. C. Behera, Scientist-‘D’ Dr. N. C. Nayak, Scientist-‘D’ Overall Supervision : Dr Utpal Gogoi, Regional Director Shri D. P. Pati, ex-Regional Director Dr. N. C. -
Dated : 10/2/2012
Dated : 10/2/2012 CIN Company Name U35303MH2007PTC175024 . ABS AVIATION AND LOGISTICS PRIVATE LIMITED U92190MH2007PTC174851 . FINE LINE MEDIA PRIVATE LIMITED U65910UP1992PTC014155 ..KALRA INVESTMENTS COMPANY PRIVATE LIMITED U24232DL2006PTC155850 .ACROSS LABORATORIES PRIVATE LIMITED U67110UP2006NPL032501 .RURAL DEVELOPMENT MICRO CREDIT SERVICES U55101PN2008PTC132986 'P SQUARE RESTAURANTS INDIA PRIVATE LIMITED .' U30000MH1996PTC103128 "NIORE" ENTREPRENEURS PRIVATE LIMITED U45202PB1959NPL002286 "THE GORAYA REGISTERED FACTORY OWNERS' ASSOCIATION" U00893BR2001PTC009521 1 INFO-MANAGEMENT SOLUTIONS PRIVATE LIMITED U72900AP2008PTC060114 1 STEP INFO TECHNOLOGIES PRIVATE LIMITED U72300AP2008PTC060633 18 STEPS TECHNOLOGIES PRIVATE LIMITED U45200AP2006PLC052141 21ST CENTURY INFRA-HEIGHTS (INDIA) LIMITED U93000AP2007PTC055417 21ST CENTURY CONCRETE WORKS PRIVATE LIMITED U45200AP1986PTC006450 21ST CENTURY CONSTRUCTIONS PVT.LTD. U64202KA2006PTC038768 21ST CENTURY INFORMATION TECHNOLOGY PRIVATE LIMITED U70102AP2006PTC048945 21ST CENTURY INVESTMENT AND PROPERTIES PVT LTD U70101OR2008PTC009794 21ST CENTURY MARKETING & DEVELOPER PRIVATE LIMITED U72200AP2007PTC054953 21ST CENTURY TECHPILLARS PRIVATE LIMITED U60200AP2007PTC054591 21ST CENTURY TRANSLINES PRIVATE LIMITED U51909KA2005PTC037456 24 X 7 ENTERPRISE SYSTEMS INTEGRATORS (INDIA) PRIVATE LIMITED U72200AP2006PTC049772 24 X 7 INFO SOLUTIONS PRIVATE LIMITED U52393KA2006PTC041298 24K RETAILING PRIVATE LIMITED U72900AP2008PTC061164 24X7 IT SERVICES PRIVATE LIMITED U72200AP2007PTC054765 24X7 SUPPORTSTATION -

1113Fe5995097fbf7370d3ad1c7
Available on line at: ISSN: 2354-5844 (Print) http://ijwem.ulm.ac.id/index.php/ijwem ISSN: 2477-5223 (Online) Geo Dynamics, Salinity Gradient, and Vegetation Interdependence in Chilika Lagoon, a Tropical Wetland in Eastern India Siba Prasad Mishra1, Sujit Kumar Mishra2, Kalptaru Das1 1 Dept. of Civil Engineering, Centurion University of Technology and Management, Bhubaneswar, Odisha, India 2 Wetland Research and Training Center, Chilika Development Authority, Balugaon, Odisha, India ABSTRACT Chilika a shallow brackish lagoon, India, is shrinking for sediment surplus budget. South Mahanadi deltaic branches i.e. Daya and Bhargavi terminate at the southwest swamps of the Lagoon. The annual average salinity of the lake was depleted from 22.31 ppt (1957-58) to 8.5 ppt. (1999-2000) as the mixing process of saline and fresh water was influenced from 1995. Trepidation of conversion of Chilika to a atrophied fresh water lake due to blooming population and their hydrologic interventions like Kolleru lake in (India), Aral Lake (Uzbekistan) was apprehended by 1950’s and was alarming by 1999 when the shallow inlet(s) shifted extreme north. The shallow mud flats of lean salinity were reclaimed further for agriculture. The ecology and biodiversity degraded with substantial pecuniary loss to the lagoon dependents. Anthropogenic interventions like, Hirakud Dam (1956), dredging of Sipakuda Inlet (2000), Naraj barrage at delta head (2004), Gobardhanpur barrages (1998) and Gabkund cut with weir (2014) were made to the hydraulic system. The deteriorating health, perturbed biodiversity and declined ecosystem of the lagoon has forced to have a comparative study of the various morphologic changes passed over the Chilika with time. -

Reconstruction of the 2003 Daya River Flood, Using Multi-Resolution and Multi-Temporal Satellite Imagery
Reconstruction of the 2003 Daya River Flood, using Multi-resolution and Multi-temporal satellite imagery Oinam Bakimchandra January, 2006 Reconstruction of 2003 Daya River Flood, using Multi- resolution and Multi-temporal Satellite Imagery by Oinam Bakimchandra Thesis submitted to the International Institute for Geo-information Science and Earth Observation in partial fulfilment of the requirements for the degree of Master of Science in Geo-information Science and Earth Observation, Specialisation: Hazard and Risk Analysis Thesis Assessment Board Thesis Supervisors: Chairman: Prof. Dr. Freek van der Meer, ITC Dr. V. Hari Prasad, IIRS External Examiner: Dr. S.K.Jain, NIH, Roorkee Drs.Dinand Alkema, ITC IIRS Member : Dr. S.P. Aggarwal,IIRS Mr.G.Srinivasa Rao, NRSA IIRS Member : Dr.V.Hari Prasad, IIRS iirs INDIAN INSTITUTE OF REMOTE SENSING (NATIONAL REMOTE SENSING AGENCY) DEPARTMENT OF SPACE, DEHRADUN, INDIA & INTERNATIONAL INSTITUTE FOR GEO-INFORMATION SCIENCE AND EARTH OBSERVATION ENSCHEDE, THE NETHERLANDS Dedicated to MY BELOVED MOTHER & FATHER I certify that although I may have conferred with others in preparing for this assignment, and drawn upon a range of sources cited in this work, the content of this thesis report is my original work. Signed ……………………. Disclaimer This document describes work undertaken as part of a programme of study at the International Institute for Geo-information Science and Earth Observation. All views and opinions expressed therein remain the sole responsibility of the author, and do not necessarily represent those of the institute. Abstract Floods are a common disaster in many parts of the world. It is considered to be the most common, costly and deadly of all natural hazards. -
PROCEEDINGS of the MEETING of STATE LEVEL EXPERT APPRAISAL COMMITTEE, ODISHA HELD on 16Th MARCH, 2021
PROCEEDINGS OF THE MEETING OF STATE LEVEL EXPERT APPRAISAL COMMITTEE, ODISHA HELD ON 16th MARCH, 2021 The SEAC met on 16th March, 2021 at 11:00 AM through video conferencing in Google Meet under the Chairmanship of Sri. B.P. Singh. The following members were present in the meeting. 1. Sri. B. P. Singh - Chairman 2. Dr. D. Swain - Member 3. Prof. (Dr.) P.K. Mohanty - Member (through VC) 4. Prof. (Dr.) H.B. Sahu - Member (through VC) 5. Sri. J. K. Mahapatra - Member 6. Sri. K. R. Acharya - Member 7. Prof. (Dr.) B.K. Satpathy - Member (through VC) 8. Dr. Sailabala Padhi - Member (through VC) 9. Dr. K.C.S Panigrahi - Member (through VC) 10. Dr. Sanjay Kumar Patnayak - Member (through VC) The agenda-wise proceedings and recommendations of the committee are detailed below. ITEM NO. 01 PROPOSAL FOR ENVIRONMENTAL CLEARANCE FOR KALYANPUR-A AND B SAND BED MINES CLUSTER ON RIVER KUAKHAI OVER AN AREA OF 34.475 HA IN VILLAGE KALYANPUR, TAHASIL –BHUBANESWAR, DISTRICT - KHORDHA, ODISHA OF TAHASILDAR, BHUBANESWAR (UNDER CLUSTER APPROACH) - TOR 1. The proposal was considered by the committee to determine the “Terms of Reference (ToR)” for undertaking detailed EIA study for the purpose of obtaining environmental clearance in accordance with the provisions of the EIA Notification, 2006 and amendment thereafter 2. The project falls under category “B” or activity 1(a) - Mining of Minerals projects under EIA Notification dated 14th September 2006 as amended from time to time. 3. The proposed project is a sand mining project under cluster approach over an area of 34.475 Ha on Kuakhai River at village- Kalyanpur, Tahasil- Bhubaneswar, Dist- Khordha, Odisha. -
Chapter II Performance Audit Department of Water Resources
Chapter II: Performance Audit Chapter II Performance Audit Department of Water Resources 2.1 Performance Audit on Contract Management Executive Summary Department of Water Resources (DoWR) has the mandate to plan, develop and manage water resources. Allocations were regularly provided in annual budget for construction and maintenance of irrigation and flood control projects. The works were carried out by contractors through contracts enforceable by law as per Indian Contracts Act of 1872. DoWR followed the provisions of Odisha Public Works Department (OPWD) Code which laid down the procedure for award and execution of works. This report focuses on the performance of DoWR in respect of tendering process and execution of projects and management of contracts. Performance Audit was conducted from April to June 2017 covering award of contracts for execution of works during 2012-17. Out of 44 divisions, 15 divisions were selected based on expenditure incurred. Against 2892 contracts valuing` 3140.93 crore (in the 15 test checked divisions), 1014 contracts with value of ` 1933.41 crore, were reviewed during the audit. Contracts were awarded to Orissa Construction Corporation (OCC) on nomination basis. A total of 198 works for a value of ` 1549.09 crore were entrusted to OCC by the DoWR bypassing the tendering procedure. Out of 198, Ninety Six (96) works awarded to OCC were not completed within the scheduled time. Of those, 51 works were delayed by more than one year. The works involving 618 contracts awarded to contractors other than OCC were not completed within the stipulated period. E-tendering portal introduced in January 2009 is yet to be fully utilised.