Perspectives Haei MAGAZINE · ISSUE 1/2021
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Modelo De Projeto De
Moção de louvor - 8k2yazhx Estado de Mato Grosso Assembleia Legislativa Despacho NP: 8k2yazhx SECRETARIA DE SERVIÇOS LEGISLATIVOS 20/08/2019 Moção de louvor nº 1216/2019 Protocolo nº 6630/2019 Autor: Dep. Dr. Eugênio Com fulcro no Art. 185-A, do Regimento Interno desta Casa de Leis, requeiro à Mesa Diretora, ouvido o Soberano Plenário, que registre nos anais "MOÇÃO DE LOUVOR", na forma: "A ASSEMBLÉIA LEGISLATIVA DO ESTADO DE MATO GROSSO, por seus membros, mediante requerimento do Deputado Dr. Eugênio, vem apresentar MOÇÃO DE LOUVOR ao Senhor FELIPE FERREIRA LIMA, atleta olímpico, Cuiabano, que em vários campeonatos internacionais de natação tem levado o nome do Brasil ao pódio." JUSTIFICATIVA O Sr. FELIPE FERREIRA LIMA, é natural de Cuiabá-MT, nasceu em 05/04/1985, filho de Helio Lima da Silva e Wilma Joaquim Ferreira Bigio. Atleta Mato-grossense, Felipe Lima, que desde os oito anos, praticava esportes como ginástica artística, lutas e futebol. Morava ao lado da Universidade Federal de Mato Grosso (Escolinha do Uirapuru/UFMT) e, lá, aos 12 anos, começou a praticar natação por recomendação de um médico, que percebeu seu crescimento acelerado. Aos 15 anos, participou do primeiro campeonato brasileiro, quando ganhou sua primeira prova dos 100 metros nado peito, e se profissionalizou. Felipe Lima foi vice-campeão mundial na prova dos 50 metros nado peito no Campeonato Mundial de Esportes Aquáticos - Gwangju 2019 em Gwangju na Coréia do Sul. A competição aconteceu entre os dias 22-28 de julho. Neste mês de agosto o atleta foi vice-campeão na prova dos 4 x 100 metro Medley, nos Jogos Pan-Americanos - Lima 2019, oficialmente denominados XVIII Jogos Pan-Americanos; essa foi a 4ª participação do nadador em jogos Pan Americanos. -

DISSERTAÇÃO JEFFERSON.Pdf
UNIVERSIDADE DE CUIABÁ PROGRAMA DE PÓS-GRADUAÇÃO STRICTO SENSU EM ENSINO TRAJETÓRIA COMPETITIVA DO ATLETA OLÍMPICO CUIABANO DE NATAÇÃO FELIPE LIMA JEFFERSON CARVALHO NEVES CUIABÁ – MATO GROSSO 2020 JEFFERSON CARVALHO NEVES TRAJETÓRIA COMPETITIVA DO ATLETA OLÍMPICO CUIABANO DE NATAÇÃO FELIPE LIMA Dissertação de Mestrado apresentada ao Programa de Pós- Graduação Stricto Sensu, Mestrado Acadêmico em Ensino na Universidade de Cuiabá (Programa Associado ao Instituto Federal de Educação, Ciência e Tecnologia de Mato Grosso - UNIC/IFMT), como parte do requisito para obtenção do Título de Mestre em Ensino, Área de Concentração: Ensino, Currículo e Saberes Docentes e da Linha de Pesquisa 2: Fundamentos Teóricos e Metodológicos da Educação Escolar, sob a orientação da Professora Dr.ª Cleonice Terezinha Fernandes CUIABÁ/MT 2020 Dados internacionais de Catalogação na Publicação (CIP) Ficha catalográfica elaborada pela Biblioteca UNIC N518t NEVES, Jefferson Carvalho Trajetória competitiva do atleta olímpico cuiabano de natação Felipe Lima / Jefferson Carvalho Neves – Cuiabá, MT 2020 94 p.: il. Dissertação (Mestrado) – Programa de Pós-graduação stricto sensu, em Mestrado como parte do requisito parcial para obtenção do título de Mestre em Ensino, área de Concentração: Ensino, Currículo e Saberes Docentes e da Linha de Pesquisa: Fundamentos Teóricos e Metodológicos da Educação Escolar. Universidade de Cuiabá – UNIC, 2020. Orientadora: Prof.ª Drª. Cleonice Terezinha Fernandes 1. Ensino. 2. Alta Performance. 3. Formação Profissional. 4. Educação Fisica. CDU: 796:797.2 Terezinha de Jesus de Melo Fonseca - CRB1/3261 SECRETARIA DE PESQUISA E PÓS-GRADUAÇÃO MESTRADO EM ENSINO ATA DE DEFESA Aos 14 dias do mês de dezembro do ano de 2020, na Universidade de Cuiabá, às 9 horas, reuniu-se a Banca Examinadora, composta por 1. -

Atletas Militares Medalhistas Pan-Americano Toronto 2015
ATLETAS MILITARES MEDALHISTAS PAN-AMERICANO TORONTO 2015 COLOCAÇÃO MODALIDADE NOME FORÇA Ouro Prata Bronze 3SG HENRIQUE RODRIGUES 200 MEDLEY EB X REV 4X100 MEDLEY MASCULINO ARTHUR MENDES MARCELO CHIERIGHINI EB X SGT GUILHERME GUIDO EB FELIPE FRANÇA FELIPE LIMA 3SG ETIENE MEDEIROS 100 COSTAS MB X SGT GUILHERME GUIDO EB 100 COSTAS EB X REV FEMININO 4X200 LIVRE JESSICA CAVALHEIRO NATAÇÃO 3 SGT LARISSA MARTINS EB EB X 3 SGT MANIELLA LYRIO EB 3SGT JOANNA MARANHÃO EB GABRIELLE GONCALVES 3SG EB ETIENE MEDEIROS 50 LIVRE MB X SG LEONARDO DE DEUS 400 METROS LIVRE EB X REV 4X100 MEDLEY FEMININO 3SGT DAYNARA DE PAULA EB 3 SGT LARISSA MARTINS EB EB /MB X 3 SGT ETIENE MEDEIROS MB JHENNIFER CONCEIÇÃO 3SGT NATALIA DE LUCCAS EB CEL JULIO ALMEIDA FAB X TIRO TIRO MAJ Cassio Cesar RIPPEL EB X Women 49erFX SGT Martine Soffiatti Grael MB X VELA KUNZE Kahena Women Laser Radial MB X 3SG Fernanda DEMETRIO DECNOP ADRIANA DA SILVA EB X MARATONA REV 4X100 GABRIEL CONSTANTINO JUNIOR DA SILVA BRUNO DE BARROS EB EB / FAB X VITOR DOS SANTOS FAB ATLETISMO GUSTAVO DOS SANTOS SGT JULIANA PAULA DOS SANTOS 5000M FAB X SGT KEILA COSTA SALTO TRIPLO EB X JUCILENE DE LIMA DARDO EB X Vanessa Espinola - Heptatlon MB X JULIO CESAR DE OLIVEIRA - Dardo FAB X RONALD JULIÃO - Disco FAB X EQUIPE MASCULINA MARCUS DALMEIDA TIRO COM ARCO FAB X SGT DANIEL REZENDE XAVIER SGT BERNARDO OLIVEIRA SGT FELIPE KITADAI EB X SGT CHARLES KOSHIRO CHIBANA EB X SGT TIAGO HENRIQUE DE OLIVEIRA CAMILO EB X SGT VICTOR RODRIGUES PENALBER DE EB X OLIVEIRA SGT LUCIANO CORREA X EB JUDÔ JUDÔ 3SG ÉRIKA DE SOUSA MIRANDA MB X 3SG RAFAELA LOPES SILVA MB X 3SG MARIANA DOS SANTOS SILVA MB X 3SG NATHALIA CASTELAN BRIGIDA MB X 3SG MARIA DE LOURDES MAZZOLENI PORTELA MB X 3SG MAYRA AGUIAR MB X 3SG MARIA SUELEN ALTHEMAN MB X 3SG Davi ALBINO MB X LUTAS ASSOCIADAS 3SG ALINE FERREIRA MB X 3 SG JOICE SOUZA MB X SGT FELIPE ALMEIDA WU EB X TIRO TC EMERSON DUARTE EB X SGT LEONARDO DE DEUS 200M BORBOLETA EB X SGT JOANNA MARANHAO 200M EB X BORBOLETA REV. -

FINA Champions Swim Series 2019 - Athletes List
Published on fina.org - Official FINA website (//www.fina.org) FINA Champions Swim Series 2019 - Athletes List Updated on: 19.04.2019 Guangzhou (CHN) - Men 50m Freestyle - USA Anthony Ervin - GBR Ben Proud - ITA Andrea Vergani - RUS Vladimir Morozov 100m Freestyle - RUS Vladimir Morozov - BEL Pieter Timmers - RUS Kliment Kolesnikov - RSA Chad Le Clos 200m Freestyle - CHN Sun Yang - RSA Chad Le Clos - LTU Danas Rapsys - CHN Wang Shun 400m Freestyle - ITA Gabriele Detti - CHN Sun Yang - AUS Jack McLoughlin - UKR Mykhailo Romanchuk 50m Backstroke - RUS Kliment Kolesnikov - ROU Robert Glinta - RUS Vladimir Morozov - USA Michael Andrew 100m Backstroke - CHN Xu Jiayu - RUS Kliment Kolesnikov - JPN Ryosuke Irie - ROU Robert Glinta 200m Backstroke - CHN Xu Jiayu - JPN Ryosuke Irie - LTU Danas Rapsys - CHN Li Guangyuan 50m Breaststroke - BRA Joao Gomes Jr - ITA Fabio Scozzoli - BRA Felipe Lima - USA Michael Andrew 100m Breaststroke - RUS Anton Chupkov - NED Arno Kamminga - ITA Fabio Scozzoli - USA Michael Andrew 200m Breaststroke - KAZ Dmitry Balandin - RUS Anton Chupkov - JPN Ippei Watanabe - CHN Qiu Haiyang 50m Butterfly - GBR Ben Proud - BRA Nicholas Santos - UKR Andriy Govorov - USA Michael Andrew 100m Butterfly - RSA Chad Le Clos - RUS Andrei Minakov - USA Michael Andrew - CHN Li Zhuhao 200m Butterfly - JPN Masato Sakai - RSA Chad Le Clos - CHN Li Zhuhao - CHN Wang Zhou 200m Ind. Medley - CHN Wang Shun - CHN Qin Haiyang - USA Michael Andrew - CHN Wang Yizhe Guangzhou (CHN) - Women 50m Freestyle - DEN Pernille Blume - SWE Sarah Sjostrom - NED -

Men's All-Time World Performers-Performances Rankings
Men’s All-Time World Performers-Performances Rankings Page 1 of 127 50 METER BACKSTROKE Top 2660 Performances 24.04** Liam Tancock, GBR 13th World Championships Rome 08-02-09 (Reaction Time: +0.60. (Note: Great Britain’s first male backstroke gold-medalist [50, 100, 200]. Tancock’s first international gold/second world- record. (Note: bronze medalist [2005, Montreal; ’07, Melbourne]) 24.07*# Camille Lacourt, FRA XXX European Championships Budapest 08-12-10 (Reaction Time: +0.74. (Nore: also clocked European-record/history’second-fastest 100 back en route to gold several days earlieir [52.11]) 24.08sf1 Tancock 13th World Championships Rome 08-01-09 (Reaction Time: +0.57) 24.23 Lacourt 16th World Championships Kazan 08-09-15 (Reaction Time: +0.68, gold medalist) 24.24a Junya Koga, JPN 13th World Championships Rome 08-02-09 (Reaction Time: +0.50. (Note: won 100 back gold in an Asian-record 52.26 clocking several days earlier.) 24.27sf2 Lacourt 16th World Championships Kazan 08-08-15 (Reaction Time: +0.69) 24.28 Koga 17th Asian Games Incheon 09-21-14 (Reaction Time: +0.52 [fastest of race]. (Note: Games record, Koga’s third-consecutive gold/record. Won @ Doha in 2K6 [25.40]; Guangzhou, 2K10 [25.08]) 24.29sf2 Koga 13th World Championships Rome 08-01-09 (Reaction Time: +0.48) 24.30sf1 Lacourt XXX European Championships Budapest 08-11-10 (Reaction Time: +0.71) 24.33* Randall Bal, USA/Stanford Eindhoven Swim Cup Eindhoven 12-05-08 (Reaction Time: +0.66) 24.34* Gerhard Zandberg, RSA/Arizona 13th World Championshps Rome 08-02-09 (Note: African record.) 24.36 Lacourt FRA Nationals/WCTs Strasbouug 03-27-11 (Note: French Open-“All Comers” record.) 24.37 Lacourt FRA Nats./Euro. -
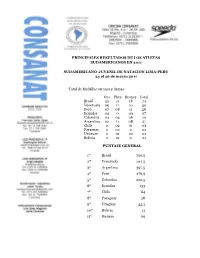
Principales Resultados De Los Atletas Sudamericanos En 2011
PRINCIPALES RESULTADOS DE LOS ATLETAS SUDAMERICANOS EN 2011 SUDAMERICANO JUVENIL DE NATACION LIMA-PERU 23 al 26 de marzo 2011 Total de Medallas varones y damas: Oro Plata Bronce Total Brasil 35 21 18 74 Venezuela 09 11 10 30 Perú 07 08 11 26 Ecuador 04 0 03 07 Colombia 03 04 06 13 Argentina 02 11 08 21 Chile 0 02 01 03 Paraguay 0 02 0 02 Uruguay 0 01 02 03 Bolivia 0 01 0 01 PUNTAJE GENERAL 1º Brasil 750,5 2º Venezuela 404,5 3º Argentina 397,5 4º Perú 376,5 5º Colombia 222,5 6º Ecuador 133 7º Chile 84 8º Paraguay 58 9º Uruguay 44,5 10º Bolivia 11 11º Guyana 09 CAMPEONATO MUNDIAL – SHANGAI/CHINA 24 a 31 de julio de 2011 Medallas De Oro: Cesar Cielo Fº Brasil - 50m Mariposa Y 50m Libre Felipe França Brasil - 50m Pecho 4º Lugar Cesar Cielo Fº Brasil - 100m Libre Kristel Kobrich Chile - 1500m Libre 5º Lugar Bruno Fratus Brasil - 50m Libre 6º Lugar Thiago Pereira Brasil - 200m Combinado Individual 12º Lugar Andreína Pinto 400m Libre RECORD Sudamericano En el cuadro general de medallas: 1º Lugar EUA 2º Lugar China 3º Lugar Brasil JUEGOS PANAMERICANOS DE GUADALAJARA 15 A 22 DE Octubre de 2011. Fueron muy importantes para los países sudamericanos, con excelentes resultados: ORO: Albert Subirats Venezuela - 100m Mariposa Kristel Kobrich Chile - 800m Libre Brasil - 200m y 400m Combinado Ind. Thiago Pereira 100m y 200m Espalda Cesar Cielo Brasil - 50m Libre y 100m Libre Felipe França Brasil - 100m Pecho Leonardo de Deus Brasil - 200m Mariposa Brasil Relevo 4 x 100m Libres varones Brasil Relevo 4 x 100m Combinado varones PLATA: Omar Pinzón Colombia – 200m Espalda Andreína Pinto Venezuela – 400m Libre Bruno Fratus Brasil - 50m Libre Felipe Lima Brasil – 100m Pecho Gracielle Herman Brasil – 50m Libre Daynara Ferreira de Paula Brasil – 100m Mariposa Joanna Maranhão Brasil – 400m Combinado Indiv. -

BTC Member's Tournament Begins
22 Wednesday, July 26, 2017 SPORTS Peaty again breaks Ledecky 50m world record Budapest “I’ve learned from the ritain’s Adam Peaty broke experience that I’ve had the last the men’s 50m breaststroke two years. “I came in tonight, I Bworld record for the second time actually thought I’d go slower as in just a few hours yesterday by I was a little less energetic, but clocking 25.95 seconds in the tomorrow’s the day to do it.” wins semi-finals. Having won the 100m The 22-year-old had broken breaststroke gold on Monday, his own record, set at the 2015 Peaty showed his blistering form world championships in Kazan, over the shorter distance by when he swam 26.10secs in the breaking the 26-second barrier. morning’s heats, with the final to The scorching time left his come on Wednesday. rivals in his wake as Brazil’s historic “This morning prepared me to Felipe Lima was 0.73secs back, do a world record again, it was while South Africa’s Cameron just me easing through the stroke, van der Burgh was third fastest touching the wall, progressing to into the final at 0.79. US Katie Ledecky the semi-final,” said Peaty. “It’s impressive eh, he’s the celebrates on the “It was the same tonight, I was first one in history (to go under podium on such a massive high from this 26 seconds),” said Van der Burgh. 12th gold morning. (AFP) Budapest championships history as she passed these championships -- from a possible atie Ledecky of the United compatriot Missy Franklin, who has six she could finish with -- and she led States made history yesterday by 11 world titles. -

Women's 1500M Free (Heat A) Final
Women's 1500m Free (Heat A) Final 2019 TYR Pro Swim Richmond | April 13, 2019 World Record: 15:20.48 Katie Ledecky, USA, Indianapolis, USA, 5/16/2018 (28.09, 58.50, 1:29.26, 2:00.25, 2:31.11, 3:02.50, 3:33.71, 4:04.88, 4:35.86, 5:06.82, 5:37.52, 6:08.29, 6:39.28, 7:10.13, 7:40.88, 8:11.70, 8:42.52, 9:13.20, 9:43.96, 10:14.83, 10:45.43, 11:16.15, 11:47.05, 12:17.94, 12:48.62, 13:19.43, 13:50.27, 14:20.71, 14:51.26, 15:20.48) American Record: 15:20.48 Katie Ledecky, USA, Indianapolis, USA, 5/16/2018 (28.09, 58.50, 1:29.26, 2:00.25, 2:31.11, 3:02.50, 3:33.71, 4:04.88, 4:35.86, 5:06.82, 5:37.52, 6:08.29, 6:39.28, 7:10.13, 7:40.88, 8:11.70, 8:42.52, 9:13.20, 9:43.96, 10:14.83, 10:45.43, 11:16.15, 11:47.05, 12:17.94, 12:48.62, 13:19.43, 13:50.27, 14:20.71, 14:51.26, 15:20.48) U.S. Open Record: 15:20.48 Katie Ledecky, USA, Indianapolis, USA, 5/16/2018 (28.09, 58.50, 1:29.26, 2:00.25, 2:31.11, 3:02.50, 3:33.71, 4:04.88, 4:35.86, 5:06.82, 5:37.52, 6:08.29, 6:39.28, 7:10.13, 7:40.88, 8:11.70, 8:42.52, 9:13.20, 9:43.96, 10:14.83, 10:45.43, 11:16.15, 11:47.05, 12:17.94, 1 Daniella Van Den Berg National Record: 17:07.88 ARU Daniella Van Den Berg Top 10 Performers This Year (2019 Calendar Year) 2018 1. -

SEI/TRE-DF - 0610929 - Edital (Juízos/Cartórios Eleitorais) File:///C:/Users/Ariane.Brandao/Appdata/Local/Microsoft/Windows/Inet
SEI/TRE-DF - 0610929 - Edital (Juízos/Cartórios Eleitorais) file:///C:/Users/ariane.brandao/AppData/Local/Microsoft/Windows/INet... TRIBUNAL REGIONAL ELEITORAL DO DF CARTÓRIO DA ZONA ELEITORAL DO EXTERIOR - ZZ SHIS, QI 13, Lote i - Bairro Lago Sul - CEP 71635-000 - Brasília - DF (61) 3048-1770 EDITAL Nº 68 - TRE-DF/PR/VPCRE/SCE/CE ZZ PARA CIÊNCIA DOS INTERESSADOS Requerimentos de Alistamento Eleitoral Indeferido (Com prazo de 05 dias para recurso nos termos do art. 17, §1º da Res. 21.538/2003 TSE) O MM. Juiz da 1ª Zona Eleitoral do Exterior/ZZ, Dr. LUIS CARLOS DE MIRANDA, no uso de suas atribuições legais, TORNA PÚBLICA, a todos quanto o presente edital virem ou dele tiverem ciência, a relação dos pedidos de alistamento, transferência e revisão, referentes aos lotes 113 a 115, INDEFERIDOS automaticamente pelo sistema ELO, o qual gerencia o Cadastro Eleitoral, por decurso do prazo de 120 dias, considerando-se que os eleitores não compareceram nesse período em que os Requerimentos estiveram em diligência, para assinar os devidos formulários e confirmar suas identidades, OU no mesmo prazo não foram enviados pelas Repartições Consulares no Exterior a documentação obrigatória à finalização do atendimento do eleitor, conforme estabelecem a Lei 4.737/65 (Código Eleitoral) e Resoluções TSE 21.538/03 e 22.155/06, e INFORMA que os interessados deverão preencher novos formulários junto ao Sistema Título Net Exterior, a fim de regularizar suas situações eleitorais. Repartição Protocolo Nome Requerimento Lote Consular AMSTERDÃ 280012406192120450 ARNALDO -

FLORIDA SWIMMING RECORDS 07/11/2017 Women's LCM LSC Records Age Group Event Time Date LSC-Club Swimmer Meet
FLORIDA SWIMMING RECORDS 07/11/2017 Women's LCM LSC Records Age Group Event Time Date LSC-Club Swimmer Meet 10 & under 50 FR 29.34 06/01/1982 FL-GSAC Jennifer Yarnall 10 & under 50 FR 29.34 01/14/2017 FL-AAC Ashlin Cannella 2017 FL CAT Al Soltis Memorial Meet 10 & under 100 FR 1:03.53 07/19/2001 FL-GCST Chelsea L.Franklin 10 & under 200 FR 2:17.54 07/25/2001 FL-GCST Chelsea L.Franklin 10 & under 400 FR 4:45.42 07/17/2003 FL-YTO Jennifer Yazbec 10 & under 50 BK 34.40 07/17/2008 FL-SYS Shealyn Atkins 2008 FL Swimming JO LC Champio 10 & under 100 BK 1:13.55 07/19/2015 FL-GSC Sydney Kang 2015 FL Swimming Summer FLAGS 10 & under 50 BR 36.87 06/01/1992 FL-BDO Jilen Siroky 10 & under 100 BR 1:20.85 07/15/2016 FL-SYS Gracie Weyant 2016 FL Swimming Summer FLAGS 10 & under 50 FL 31.66 07/01/1984 FL-GSAC Susan Lerch 10 & under 100 FL 1:10.71 07/17/2014 FL-SPAC Sara Stotler 2014 FLS Summer Age Group Cham 10 & under 200 IM 2:35.95 07/19/2015 FL-GSC Sydney Kang 2015 FL Swimming Summer FLAGS 10 & under 200 FR-R 2:06.07 07/15/2016 FL-STAR Ella Klyce,<br> Emma 2016 FL Swimming Summer FLAGS Sundermeyer,<br> Tabitha Driscoll,<br> Addison Reese 10 & under 200 MED-R 2:20.90 07/16/2016 FL-STAR Ella Klyce,<br> Peyton Powell,<br> 2016 FL Swimming Summer FLAGS Addison Reese,<br> Emma Sundermeyer Women's LCM LSC Records Age Group Event Time Date LSC-Club Swimmer Meet 11-12 50 FR 27.05 08/08/2015 FL-GTSA Ella Marlow Z 2015 MS 2015 Southern Zones Age Group 11-12 100 FR 58.81 07/25/2001 FL-CAT CHELSEA B.NAUTA 11-12 200 FR 2:06.31 07/30/2003 FL-GCST Chelsea Franklin -

Lista Em Ordem Alfabética
CANDIDATOS QUE TERÃO CORRIGIDAS AS PROVAS DA 2ª FASE LISTA EM ORDEM ALFABÉTICA - LETRA F - DATA: 25/02/2010 Inscrição Nome Curso 1ª Opção 0113620 FABIANA BARBOSA KAMACHE Direito 0627640 FABIANA BARBOSA NESPOLI Ciências Contábeis 0213071 FABIANA BORGES SANTIAGO Arquitetura e Urbanismo 0051756 FABIANA CARDOSO GOMES Enfermagem e Obstetrícia 0273597 FABIANA CARVALHO DE ALMEIDA Psicologia 0248401 FABIANA CHEADE HAMILTON Engenharia 0365203 FABIANA CRISTINA DE OLIVEIRA ROCHA Pedagogia 0192619 FABIANA DA SILVA Licenciatura em Educação Física 0369802 FABIANA DA SILVA MORESCHE Enfermagem e Obstetrícia 0460427 FABIANA DA SILVA VENTURA Licenciatura em Matemática 0355097 FABIANA DE CARVALHO BELCHIOR Farmácia 0765341 FABIANA DE SOUZA SAMUEL Fisioterapia 0706116 FABIANA DIONISIO NAZARETH GUERRA Serviço Social 0488283 FABIANA DOS SANTOS OLIVEIRA Medicina 0595748 FABIANA FELIX BARRETO História 0024317 FABIANA FERREIRA DE MELO Química Industrial 0201138 FABIANA FIGUEIREDO DE ALENCAR Arquitetura e Urbanismo 0755796 FABIANA GOMES DE CASTRO SEIJAS Direito 0671215 FABIANA GUTIERREZ PANOZO Medicina 0197025 FABIANA KAISSA GALLEGOS SEPULVEDA MOREIRA Química com Atribuição Tecnológica 0363960 FABIANA LINHARES MARTIM Estatística 0513547 FABIANA MAGNO DE LACERDA Biblioteconomia e Gestão de Unid. de Inf. 0211230 FABIANA MAIA SANTOS Licenciatura em Química 0428485 FABIANA MARIA DOS SANTOS Letras / Português-Inglês 0722758 FABIANA MIRANDA GOULART Defesa e Gestão Estratégica Internacional 0230111 FABIANA MIRANDA PRESTES Direito 0536393 FABIANA MORAES FERRO Defesa e -

5885 DIÁRIO OFICIAL No 4
4 DIÁRIO OFICIAL No 5885 ANO XXXIII - ESTADO DO TOCANTINS, TERÇA-FEIRA, 13 DE JULHO DE 2021 COMISSÃO DO CONCURSO PÚBLICO POLÍCIA MILITAR CONCURSO PÚBLICO PARA O INGRESSO NO CURSO DE FORMAÇÃO DE PRAÇAS (CFP) DO QUADRO DE PRAÇAS POLICIAIS MILITARES (QPPM) Portaria Nº 021/2021 - DAL/PMTO. EDITAL Nº 7 - PMTO - CFPE, DE 13 DE JULHO DE 2021 O CORONEL QOPM COMANDANTE-GERAL DA POLÍCIA MILITAR DO ESTADO DO TOCANTINS, no uso de suas atribuições legais O Coronel QOPM Marizon Mendes Marques, Presidente da Comissão do Concurso Público, torna públicos o resultado final na prova lhe conferidas pelos incisos I e IV, do §1º, do art. 42, da Constituição de redação e a convocação para o exame de capacidade física, referentes Estadual do Tocantins, de 05 de outubro de 1989, pelo art. 10, da Lei ao concurso público para o ingresso no Curso de Formação de Praças Complementar nº 128, de 14 de abril de 2021, pelo art. 75, da Lei nº 8.666 (CFP) do Quadro de Praças Policiais Militares (QPPM) da Polícia Militar de 21 de junho de 1993; do Estado do Tocantins (PMTO). Resolve: 1 DO RESULTADO FINAL NA PROVA DE REDAÇÃO 1.1 Resultado final na prova de redação, na seguinte ordem: Art. 1º DESIGNAR a 1º SGT QPPM KEILE XAVIER DE SOUZA cargo/sexo, número de inscrição, nome do candidato em ordem alfabética LINHARES, RG: 05.385/2, MAT. 70182, e o 2º SGT QPPM LUCAS e nota final na prova de redação. ALVES SILVA SANTOS, RG: 05.969/2, MAT. 81696, para exercerem, respectivamente, as atribuições de fiscal titular e fiscal substituto do 1.1.1 CARGO: ALUNO-SOLDADO QPPM/FEMININO Contrato nº 18/2021, de aquisição de aparelhos de ar-condicionado para 30000526, Acsa Nascimento Alves, 25.20/30040188, Adriana APMT, conforme o Processo nº 2020/09030/000283.