Carbon Sequestration by Victorian Inland Wetlands
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Real Challenge for Wetland Management
Hydrobiologia DOI 10.1007/s10750-012-1163-4 CLIMATE CHANGE AND AUSTRALIAN WETLANDS Review Paper A legacy of climate and catchment change: the real challenge for wetland management Peter Gell • Keely Mills • Rosie Grundell Received: 6 December 2011 / Accepted: 6 May 2012 Ó Springer Science+Business Media B.V. 2012 Abstract Wetland managers are faced with an array capacity to recover from the recent ‘big dry’. These of challenges when restoring ecosystems at risk from sedimentary archives reveal most modern wetlands to be changing climate and human impacts, especially as many outside their historical range of variability. This approach of these processes have been operating over decadal– provides a longer-term context when assessing wetland millennial timescales. Variations in the level and salinity condition and better establishes the restoration challenge of the large crater lakes of western Victoria, as revealed posed by the impact of climate change and variability and over millennia by the physical, chemical and biological human impacts. evidence archived in sediments, attest to extended periods of positive rainfall balance and others of rainfall Keywords Australia Á Climate change Á Ecological deficit. The recent declines in the depth of these lakes condition Á Wetland response Á Acceptable change have been attributed to a 15% decline in effective rainfall since AD 1859. Whilst some sites reveal state shifts following past droughts, the response of most wetlands to Introduction millennial-scale climatic variations is muted. Regional wetland condition has changed comprehensively, how- The management of wetlands has largely been under- ever, since European settlement, on account of extensive pinned by the assumption that their hydrology and catchment modifications. -

Assessing the Cost-Effectiveness of a Fish Stocking Program in a Culture
G Model FISH-4539; No. of Pages 10 ARTICLE IN PRESS Fisheries Research xxx (2016) xxx–xxx Contents lists available at ScienceDirect Fisheries Research journal homepage: www.elsevier.com/locate/fishres Full length article Assessing the cost-effectiveness of a fish stocking program in a culture-based recreational fishery a,b,∗ c d a b Taylor L. Hunt , Helen Scarborough , Khageswor Giri , John W. Douglas , Paul Jones a Fisheries Management and Science Branch, Fisheries Victoria, Department of Economic Development, Jobs, Transport and Resources, PO Box 114, Queenscliff, Victoria, 3225, Australia b Centre for Integrative Ecology, School of Life & Environmental Sciences, Faculty of Science, Engineering & Built Environment, Deakin University, PO Box 423, Warrnambool, Victoria, 3280, Australia c Department of Economics, Deakin Business School, Deakin University, PO Box 423, Warrnambool, Victoria, 3280, Australia d Biometrics Unit, Agriculture Research Division, Department of Economic Development, Jobs, Transport and Resources, 32 Lincoln Square North, Parkville, Victoria, 3053, Australia a r t i c l e i n f o a b s t r a c t Article history: Fish stocking is commonly used to enhance, create and maintain recreational fisheries that typically Received 5 February 2016 generate significant economic activity. As fish stocking can be highly popular with stakeholders and is Received in revised form 31 August 2016 often a large economic investment, it should be evaluated to ensure it provides adequate return and is an Accepted 4 September 2016 effective use of fisheries management funds. In this study we evaluated the cost-effectiveness of a fish Handled by: Chennai Guest Editor stocking program for non-native salmonid species of brown trout, rainbow trout and Chinook salmon at Available online xxx Lake Purrumbete, south-western Victoria, Australia. -

Vict.. on HIS MAJESTY's SERVICE Geological Notebook No. 9
Cover ©Gill Estate. Except as otherwise indicated, the Gill Notebooks are copyright to the Gill Estate and licensed Western Dist. – Vict.. under a Creative Commons Attribution-NonCommercial- NoDerivatives 4.0 International License. ON HIS MAJESTY’S SERVICE Geological Notebook No. 9 National Museum of Victoria, Melbourne, C.I, Victoria, Australia Edmund D Gill Title page Notebook No. 9 If found please return to – Edmund D Gill Palaeontologist National Museum Russell Street Melbourne CI. 1951 Index 1 Lake Colongulac 3 “Chocolyn” bores 7 L. Colongulac levels 9 Analyses bore water 10 Tuff & loess 12 Loess cliffs 13 Camperdown Basalt 14 Winchelsea “laterite” 17 Sewerage outlet Dennington levels 19 Nestle’s 20 Levels Dennington Stn to Moulden’s Quarry 23 Moulden’s Q 25 Auger hole near Moulden’s Q 27 Levels M’s Quarry to coast 37 Excav. S.E. of Moulden’s Quarry Kelly Swamp 39 Excav. Merri Flats 40 Auger hole, bank Merri R. 41 Auger hole near Sanitary Depot 45 Dunes near Sanitary Depot 47 Tower Hill Beach 51 Goose Lagoon 53 Lake Gillear 54 Lake Gillear auger hole 55 Port Fairy drain 57 Holloway’s Beach 61 Levels Holloway’s Beach 67 Holloway’s Cave 70 Warrnambool 71 Thunder Pt. 72 Tower Hill Beach 74 Rd. ctg. E. of Illowa 75 Goose Lagoon 77 L. Colongulac 88 Queensland Museum 89 L. Keilambete 90 L. Connewarren, Mt. Shadwell, Mt. Elephant 91 Shelford 93 L. Colongulac 102 “Puunyart” Bore 103 L. Kariah L. Terangpom 104 Garvoc 107 Lake Terang 109 Lake Elingamite 111 Formation of calderas 112 Ewan’s Hill 113 Lake Purrumbeet 118 Camperdown Quarry south of Mt Leura 119 Mt Leura Camperdown 120 Quarries Camperdown 127 Rd. -
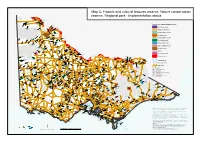
Map C: Historic and Cultural Features Reserve, Nature Conservation Reserve, Regional Park - Implementation Status
Map C: Historic and cultural features reserve, Nature conservation reserve, Regional park - implementation status Merbein South FR Psyche Bend Pumps HCF R Mildura FFR Yarrara Yatpool FFR FR Lambert Island NCR Historic and cultural features reserve Karadoc Bum bang Island Meringur NCR HCF R FFR Toltol Fully implemented FFR Lakes Powell and Carpul Wemen Mallanbool NCR FFR Nowingi Ironclad C atchment FFR and C oncrete Tank Partially implemented HR Bannerton FFR Wandown Implementation unclear Moss Tank FFR Annuello FFR FFR Bolton Kattyoong Tiega FFR Unimplemented FR FR Kulwin FFR Degraves Tank FR Kulwin Manya Wood Wood Gnarr FR Cocamba Nature conservation reserve FR Walpeup Manangatang FFR Dunstans Timberoo Towan Plains FR FFR (Lulla) FFR FFR FFR FFR Bronzewing FFR Fully implemented Murrayville FFR Chinkapook FR FFR Yarraby Chillingollah FR Koonda Nyang FFR FR Boinka FFR Lianiduck Partially implemented FR FFR Welshmans Plain FFR Dering FFR Turriff Lake Timboram FFR Waitchie FFR Implementation unclear FFR Winlaton Yetmans (Patchewollock) Wathe NCR FFR FFR Green Yassom Swamp Dartagook Unimplemented NCR Lake NCR Koondrook Paradise RP Brimy Bill Korrak Korrak HC FR FFR WR NCR Cambacanya Angels Rest Wandella Kerang RP Regional park Lake FFR FR NCR Gannawarra Red Gum Albacutya Swamp V NCR Cohuna HCFR RP Wangie FFR Tragowel Swamp Pyramid Creek Cohuna Old Fully implemented NCR Cannie NCR NCR Court House Goyura Rowland HCF R HR Towaninny NCR Flannery Red Bluff Birdcage NCR Griffith Lagoon NCR Bonegilla Unimplemented FFR NCR Bethanga FFR Towma (Lake -

Overview of the Impacts of Introduced Salmonids on Australian Native Fauna
OVERVIEW OF THE IMPACTS OF INTRODUCED SALMONIDS ON AUSTRALIAN NATIVE FAUNA by P. L. Cadwallader prepared for the Australian Nature Conservation Agency 1996 ~~ AUSTRALIA,,) Overview of the Impacts of Introduced Salmonids on Australian Native Fauna by P L Cadwallader The views and opinions expressed in this report are those of the authors and do not necessarily reflect those of the Commonwealth Government, the Minister for the Environment or the Director of National Parks and Wildlife. ISBN 0 642 21380 1 Published May 1996 © Copyright The Director of National Parks and Wildlife Australian Nature Conservation Agency GPO Box 636 Canberra ACT 2601 Design and art production by BPD Graphic Associates, Canberra Cover illustration by Karina Hansen McInnes CONTENTS FOREWORD 1 SUMMARY 2 ACKNOWLEDGMENTS 3 1. INTRODUCTION 5 2. SPECIES OF SALMONIDAE IN AUSTRALIA 7 2.1 Brown trout 7 2.2 Rainbow trout 8 2.3 Brook trout 9 2.4 Atlantic salmon 9 2.5 Chinook salmon 10 2.6 Summary of present status of salmonids in Australia 11 3. REVIEW OF STUDIES ON THE IMPACTS OF SALMONIDS 13 3.1 Studies on or relating to distributions of salmonids and native fish 13 Grey (1929) Whitley (1935) Williams (1964) Fish (1966) Frankenberg (1966, 1969) Renowden (1968) Andrews (1976) Knott et at. (1976) Cadwallader (1979) Jackson and Williams (1980) Jackson and Davies (1983) Koehn (1986) Jones et al. (1990) Lintermans and Rutzou (1990) Minns (1990) Sanger and F ulton (1991) Sloane and French (1991) Shirley (1991) Townsend and Growl (1991) Hamr (1992) Ault and White (1994) McIntosh et al. (1994) Other Observations and Comments 3.2 Studies Undertaken During the Invasion of New Areas by Salmonids 21 Tilzey (1976) Raadik (1993) Gloss and Lake (in prep) 3.3 Experimental Introduction study 23 Fletcher (1978) 3.4 Feeding Studies, Including Analysis of Dietary Overlap and Competition, and Predation 25 Introductory Comments Morrissy (1967) Cadwallader (1975) Jackson (1978) Cadwallader and Eden (1981,_ 1982) Sagar and Eldon (1983) Glova (1990) Glova and Sagar (1991) Kusabs and Swales (1991) Crowl et at. -

Purrumbete Verandah, 2008 1
Coolabah, No.11, 2013, ISSN 1988-5946, Observatori: Centre d’Estudis Australians, Australian Studies Centre, Universitat de Barcelona Purrumbete Verandah, 2008 1 Seth Keen Copyright©2013 Seth Keen. This text may be archived and redistributed both in electronic form and in hard copy, provided that the author and journal are properly cited and no fee is charged. Abstract: In describing his 2008 digital video installation, a continuous 3 minute five second loop, Seth Keen introduces a contemporary engagement with the historic landscape of rural Victoria witnessed by Von Guérard in his paintings of the 1850s. Bringing together a background in documentary practice and graphic design, Seth is interested, as a media artist, in using digital technologies to explore forms of environmental portraiture that document relationships between people and place. In this video work, he revisits the location and landscape painting, From the verandah of Purrumbete, 18582 by Eugene Von Guérard. Purrumbete Verandah, 2008 documents the landscape and location that Von Guérard painted in 1858. Video is used to record the view across Lake Purrumbete from the verandah of the Purrumbete Homestead. Edited into this shot, are shots of local fishermen and a view of the Purrumbete Homestead recorded from Picnic Point on Lake Purrumbete. Caught up in the tranquillity of this location, I slipped into the pace of the fishermen and their interaction with Lake Purrumbete. They become a pivot for differing viewpoints on the verandah location. The video work can be viewed on the web at http://www.sethkeen.net/portfolio/purrumbete-verandah/. These shots originated from the Locative Painting3 research project, which records the numerous locations Von Guérard painted in the Corangamite Shire. -

Registered Aboriginal Parties in Victoria Horse S Hoe Lagoon
!( !( WEST WYALONG D ar ling Ri ver WENTW ORTH !( Registered Aboriginal Parties in Victoria Horse S hoe Lagoon r MILDURA e v !( i Registered Aboriginal Parties* R Lake Wallawalla n a !( l h c GRIFFITH a L !( <null> Barengi Gadjin Land Council Aboriginal Corporation !( YOUNG RE D CLIFFS !( !( Mu rrumb idgee Rive Bunurong Land Council Aboriginal Corporation r TEMORA !( HAY !( !( Dja Dja Wurrung Clans Aboriginal Corporation ROBINVALE LEETON HA RDEN !( !( BALRANALD !( COOTAMUNDRA Eastern Maar Aboriginal Corporation Rocket Lake !( Lake Cantala NARRANDERA !( First People of the Millewa-Mallee Aboriginal Corporation GANMAIN Lake Kramen !( COOLAMON S U N S E T C OUNTRY !( GOULBURN MILDURA !( JUNEE Gunaikurnai Land and WYaAStSers Aboriginal Corporation NEW SOUTH WALES !( !( Gunditj Mirring Traditional Owners Aboriginal Corporation Bailey Plain Salt Pan OUYEN GUNDAGAI Lake Burrinjuck !( !( WAGGA WAGGA Taungurung Land and Waters Council Aboriginal Corporation SWAN HILL !( Wathaurung Aboriginal Corporation Lake Wahpool JERILDERIE TUMUT Lake Tiboram !( !( !( Wurundjeri Woi Wurrung Cultural Heritage Aboriginal Corporation Lake Tyrrell SWAN HILL L itt le CANBERRA M QUEANBEYAN u !( rr W !( a a E y k d R o w Lake Boga iv o a e l R rd r iv R Yorta Yorta Nation Aboriginal Corporation e i r v er DENILIQUIN Lake Tutchewop !( Blowering Reservoir Kangaroo Lake Indicates an area where more than one RAP exists Lake Charm Lake Cullen e.g. Eastern Maar Aboriginal Corporation and B I G D E SERT The Marsh !( FINLEY G o Gunditj Miro ring Traditional Owners Aboriginal Corporation d r r a d e i v KERANG g i b R !( Talbingo Reservoir e e e R e GANNAW ARRA g iv d P e i r b y r Tantangara Reservoir Lake A lbacutya a COHUNA m TOCUMWAL u m r !( r i d !( u !( C M r e * This map illustrates all Registered Aboriginal Parties on e k COBRAM FEBRUARY 6, 2020. -

Redfin Perch in Lake Purrumbete – Managing the Fishery Recreational Fishing Grants Program Research Report
Redfin perch in Lake Purrumbete – managing the fishery Recreational Fishing Grants Program Research Report Redfin perch in Lake Purrumbete – managing the fishery June 2018 Recreational Fishing Grants Program Research Report Published by the Victorian Government, Victorian Fisheries Authority (VFA), June 2018 © The State of Victoria, Victorian Fisheries Authority, Melbourne, June 2018 This publication is copyright. No part may be reproduced by any process except in accordance with the provisions of the Copyright Act 1968. Authorised by the Victorian Government, 1 Spring Street, Melbourne. Preferred way to cite this publication: VFA (2017) Redfin perch in Lake Purrumbete – managing the fishery. Recreation Fishing Grants Program Research Report. ISBN 978-1-925733-43-3 (Print) ISBN 978-1-925733-44-0 (pdf/online) Copies are available by emailing [email protected] For more information contact the VFA Customer Service Centre 136 186 Disclaimer This publication may be of assistance to you but the State of Victoria and its employees do not guarantee that the publication is without flaw of any kind or is wholly appropriate for your particular purposes and therefore disclaims all liability for any error, loss or other consequence which may arise from you relying on any information in this publication. Accessibility If you would like to receive this publication in an accessible format, such as large print or audio, please telephone 136 186, or email [email protected] Deaf, hearing impaired or speech impaired? Call us -

Highlighting the Role of Groundwater in Lake– Aquifer Interaction to Reduce Vulnerability and Enhance Resilience to Climate Change
Article Highlighting the Role of Groundwater in Lake– Aquifer Interaction to Reduce Vulnerability and Enhance Resilience to Climate Change Yohannes Yihdego 1,2,*, John A Webb 2, and Babak Vaheddoost 3 1 Snowy Mountains Engineering Corporation (SMEC), Sydney, New South Wales 2060, Australia 2 Environmental Geoscience, La Trobe University, Melbourne, Victoria 3086, Australia; [email protected] 3 Hydraulic Lab., Istanbul Technical University, Istanbul 34467, Turkey; [email protected] * Correspondence: [email protected] Academic Editor: Abdon Atangana Received: 22 December 2016; Accepted: 8 February 2017; Published: 13 February 2017 Abstract: A method is presented to analyze the interaction between groundwater and Lake Linlithgow (Australia) as a case study. A simplistic approach based on a “node” representing the groundwater component is employed in a spreadsheet of water balance modeling to analyze and highlight the effect of groundwater on the lake level over time. A comparison is made between the simulated and observed lake levels over a period of time by switching the groundwater “node “on and off. A bucket model is assumed to represent the lake behavior. Although this study demonstrates the understanding of Lake Linlithgow’s groundwater system, the current model reflects the contemporary understanding of the local groundwater system, illustrates how to go about modeling in data‐scarce environments, and provides a means to assess focal areas for future data collection and model improvements. Results show that this approach is convenient for getting first‐hand information on the effect of groundwater on wetland or lake levels through lake water budget computation via a node representing the groundwater component. -

Assessing the Cost-Effectiveness of a Fish Stocking Program in a Culture-Based Recreational Fishery
Fisheries Research 186 (2017) 468–477 Contents lists available at ScienceDirect Fisheries Research j ournal homepage: www.elsevier.com/locate/fishres Full length article Assessing the cost-effectiveness of a fish stocking program in a culture-based recreational fishery a,b,∗ c d a b Taylor L. Hunt , Helen Scarborough , Khageswor Giri , John W. Douglas , Paul Jones a Fisheries Management and Science Branch, Fisheries Victoria, Department of Economic Development, Jobs, Transport and Resources, PO Box 114, Queenscliff, Victoria, 3225, Australia b Centre for Integrative Ecology, School of Life & Environmental Sciences, Faculty of Science, Engineering & Built Environment, Deakin University, PO Box 423, Warrnambool, Victoria, 3280, Australia c Department of Economics, Deakin Business School, Deakin University, PO Box 423, Warrnambool, Victoria, 3280, Australia d Biometrics Unit, Agriculture Research Division, Department of Economic Development, Jobs, Transport and Resources, 32 Lincoln Square North, Parkville, Victoria, 3053, Australia a r a t i b s c t l e i n f o r a c t Article history: Fish stocking is commonly used to enhance, create and maintain recreational fisheries that typically Received 5 February 2016 generate significant economic activity. As fish stocking can be highly popular with stakeholders and is Received in revised form 31 August 2016 often a large economic investment, it should be evaluated to ensure it provides adequate return and is an Accepted 4 September 2016 effective use of fisheries management funds. In this study we evaluated the cost-effectiveness of a fish Handled by: Chennai Guest Editor stocking program for non-native salmonid species of brown trout, rainbow trout and Chinook salmon at Available online 11 September 2016 Lake Purrumbete, south-western Victoria, Australia. -

The Macrobenthic Fauna of Great Lake and Arthurs Lake, Tasmania
THE MACROBENTHIC FAUNA OF GREAT LAKE AND ARTHURS LAKE, TASMANIA. by ea W. FULTON B.Sc. Submitted in partial fulfilment of the requirements for the degree of Master of Science UNIVERSITY OF TASMANIA HOBART May 1981 Old multiple-arch dam, Miena Great Lake, 1962 (Photo courtesy P. A. Tyler) "Benthic collecting can be fun ...." To the best of my knowledge, this thesis does not contain any material which has been submitted for any other degree or diploma in any university except as stated therein. Further, I have not knowingly included a copy or paraphrase of previously published or written material from any source without due reference being made in the text of this thesis. Wayne Fulton iii CONTENTS Page Title 1 Statement CONTENTS iii ABSTRACT 1 CHAPTER 1 INTRODUCTION 3 1.1 AIMS 3 1.2 HISTORY 4 1.3 DRAINAGE ALTERATIONS 11 1.4 GEOLOGY 14 1.5 GEOMORPHOLOGY 15 1.6 BIOLOGICAL HISTORY 18 CHAPTER 2 METHODS 21 2.1 SAMPLING DEVICE 21 2.2 SAMPLING PROGRAM 22 2.2.1 Collection and Sorting of Samples 25 2.2.2 Sample Sites 29 2.2.3 Summary of Sampling Program 35 2.3 SITE DESCRIPTIONS, PHYSICAL AND CHEMICAL DATA 36 2.3.1 Discussion: Site Physical and Chemical Relationships .53 2.4 TREATMENT OF DATA 55 iv Page CHAPTER 3 FAUNAL COMPOSITION OF GREAT LAKE AND ARTHURS LAKE 58 3.1 INTRODUCTION 58 3.2 RESULTS 58 3.3 DISCUSSION 70 CHAPTER 4 QUANTITATIVE FAUNAL VARIATION OF GREAT LAKE AND ARTHURS LAKE 79 4.1 INTRODUCTION 79 4.2 RESULTS 80 4.2.1 Faunal Variation 80 4.2.1.1 Within series variation 81 4.2.1.2 Seasonal variation 86 4.2.1.3 Inter-site variation 93 4.2.1.3.1 -

Cp-2017-31-Supplement.Pdf
Table S1. All records identified within Australasia that span the Common Era. State refers to the political state, country, or geographic region where the record originates: NSW=New South Wales, VIC=Victoria, SA=South Australia, WA=Western Australia, NT=Northern Territory, QLD=Queensland, TAS=Tasmania, ACT=Australian Capitol Territory, TS=Torres Strait, INDO=Indonesia, NZ=New Zealand, PNG=Papua New Guinea, Pacific= Pacific Ocean Islands, ANT= Antarctica, BS=Bass Strait; Elevation is in meters above sea 5 level; Resolution refers to average number of samples per year, where indicated by the original authors or calculated from the published text. Record Name State Latitude Longitude Elevation Classification Oldest Year Youngest Year Resolution Reference Richmond River QLD -28.48 152.97 100 LakeWetland 5451YBP(+/-133) -57YBP(+/-1) NA Logan et al., 2011 Theresa/Capella Creek QLD -23.00 148.04 Various LakeWetland 791YBP(+/-69) -48YBP 10 Hughes et al., 2009 Mill Creek NSW -33.39 151.04 4 LakeWetland 10458YBP(+/-215) -40YBP NA Dodson and Thom, 1992 Mill Creek NSW -33.39 151.04 4 LakeWetland 684YBP(+/-106) -43YBP(+/-0) 7 Johnson, 2000 Yarlington Tier TAS -42.52 147.29 650 LakeWetland 10174YBP(+/-395) NA NA Harle et al., 1993 Rooty Breaks Swamp VIC -37.21 148.86 1100 LakeWetland 6249YBP(+/-309) NA NA Ladd, 1979b Diggers Creek Bog NSW -36.23 148.48 1690 LakeWetland 11817YBP(+/-573) NA 156 Martin, 1999 Nullabor - N145 SA -32.07 127.85 15 LakeWetland 24161YBP(+/-2219) NA NA Martin, 1973 Nullabor - Madura WA -31.98 127.04 27 LakeWetland 8708YBP(+/-846)