Convert Naphtha to Gasoline with Methaforming
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Reasoned Request on Failure of Bosnia Herzegovina to Comply With
ANNEX 9a/14th MC/10-08-2016 TO THE MINISTERIAL COUNCIL OF THE ENERGY COMMUNITY represented by the Presidency and the Vice-Presidency of the Energy Community REASONED REQUEST in Case ECS-2/13 Submitted pursuant to Article 90 of the Treaty establishing the Energy Community and Article 29 of Procedural Act No 2008/1/MC-EnC of the Ministerial Council of the Energy Community of 27 June 2008 on the Rules of Procedure for Dispute Settlement under the Treaty, the SECRETARIAT OF THE ENERGY COMMUNITY against BOSNIA AND HERZEGOVINA seeking a Decision from the Ministerial Council that Bosnia and Herzegovina, 1. by failing to ensure within the prescribed time limit that heavy fuel oils are not used if their sulphur content exceeds 1.00 % by mass in the entire territory of the Contracting Party, Bosnia and Herzegovina has failed to fulfil its obligations under Article 3(1) of Directive 1999/32/EC in conjunction with Article 16 of the Treaty establishing the Energy Community; 2. by failing to ensure within the prescribed time limit that gas oils are not used if their sulphur content exceeds 0.1 % by mass in the entire territory of the Contracting Party, Bosnia and Herzegovina has failed to fulfil its obligations under Article 4(1) of Directive 1999/32/EC in conjunction with Article 16 of the Treaty establishing the Energy Community. The Secretariat of the Energy Community has the honour of submitting the following Reasoned Request to the Ministerial Council. I. Relevant Facts (1) As a Contracting Party to the Treaty establishing the Energy Community (“the Treaty”), Bosnia and Herzegovina is under an obligation to implement the acquis communautaire on environment as listed in Article 16 of the Treaty. -

Naphtha Safety Data Sheet According to Regulation (EC) No
Naphtha Safety Data Sheet according to Regulation (EC) No. 1907/2006 (REACH) with its amendment Regulation (EU) 2015/830 Revision date: 29 Dec 2020 Version: 4.0 SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Product form : Substance (UVCB) Trade name : Naphtha Chemical name : Solvent naphtha (petroleum), light aliph.; Low boiling point naphtha; [A complex combination of hydrocarbons obtained from the distillation of crude oil or natural gasoline. It consists predominantly of saturated hydrocarbons having carbon numbers predominantly in the range of C5 through C10 and boiling in the range of approximately 35°C to 160°C (95°F to 320°F).] IUPAC name : Solvent naphtha (petroleum), light aliph. EC Index-No. : 649-267-00-0 EC-No. : 265-192-2 CAS-No. : 64742-89-8 REACH registration No : 01-2119471306-40 Type of product : Hydrocarbons Synonyms : Solvent naphtha (petroleum), light aliph. Product group : Raw material BIG No : F06734 1.2. Relevant identified uses of the substance or mixture and uses advised against 1.2.1. Relevant identified uses Main use category : Industrial use Use of the substance/mixture : Chemical raw material Function or use category : Intermediates Title Life cycle stage Use descriptors Use of substance as intermediate Industrial SU8, SU9, PROC1, PROC2, PROC3, PROC8a, PROC8b, (ES Ref.: ES 2) PROC15, PROC28, ERC6a Manufacture of substance Manufacture PROC1, PROC2, PROC3, PROC8a, PROC8b, PROC15, (ES Ref.: ES 1) PROC28, ERC1 Full text of use descriptors: see section 16 1.2.2. Uses advised against No additional information available NOR1.3. Details of the supplier of product safety information sheet Manufacturer Only Representative ZapSibNeftekhim LLC Gazprom Marketing and Trading France Promzona avenue des Champs-Elysées 68 626150 Tobolsk, Tyumen region - Russion Federation 75008 Paris - France T +7 (3456) 398-000 - F +7 (3456) 266-449 T +33 1 42 99 73 50 - F +33 1 42 99 73 99 [email protected] [email protected] 1.4. -

Imports of Crude Oil in Czech Republic January to September 2015
Imports of Crude Oil in Czech republic January to September 2015 Country of Origin Imports (kt) Shares (%) Azerbaijan 1 740,7 30,90 Kazakhstan 619,6 11,00 Hungary 15,3 0,27 Russia 3 257,5 57,83 Total 5 633,1 Mean retail prices of motor fuels in CZK/litre in years 2011, 2012, 2013, 2014 and 2015, monthly 2011 product 1/11 2/11 3/11 4/11 5/11 6/11 7/11 8/11 9/11 10/11 11/11 12/11 Average Regular Unleaded gasoline 91, 10 ppm 33,54 33,41 34,13 34,65 - - - - - - - - 33,93 Premium Unl. gasoline 95, 10 ppm 33,47 33,38 34,22 34,86 35,21 34,88 34,75 34,78 34,65 34,81 34,99 34,95 34,58 Super plus Unl. 98, 10 ppm 35,35 35,27 35,94 36,33 36,94 36,74 36,64 36,70 36,67 36,77 36,85 36,77 36,41 Regular Unl. 91 with AK additive 32,97 32,95 33,31 33,62 33,78 33,69 33,96 33,91 33,84 34,07 34,43 34,51 33,75 ULS Diesel, 10 ppm 32,72 32,84 33,80 34,42 34,44 34,24 34,25 34,34 34,38 34,69 35,31 35,58 34,25 LPG 17,16 17,56 17,40 17,41 17,34 17,21 17,03 16,93 16,84 16,76 16,79 17,19 17,14 Comment: Observation of Regular Unleaded Gasoline 91 have been stopped since 1.5.2011 (low market share) 2012 product 1/12 2/12 3/12 4/12 5/12 6/12 7/12 8/12 9/12 10/12 11/12 12/12 Average Premium Unl. -

Argus Nefte Transport
Argus Nefte Transport Oil transportation logistics in the former Soviet Union Volume XVI, 5, May 2017 Primorsk loads first 100,000t diesel cargo Russia’s main outlet for 10ppm diesel exports, the Baltic port of Primorsk, shipped a 100,000t cargo for the first time this month. The diesel was loaded on 4 May on the 113,300t Dong-A Thetis, owned by the South Korean shipping company Dong-A Tanker. The 100,000t cargo of Rosneft product was sold to trading company Vitol for delivery to the Amsterdam-Rotter- dam-Antwerp region, a market participant says. The Dong-A Thetis was loaded at Russian pipeline crude exports berth 3 or 4 — which can handle crude and diesel following a recent upgrade, and mn b/d can accommodate 90,000-150,000t vessels with 15.5m draught. 6.0 Transit crude Russian crude It remains unclear whether larger loadings at Primorsk will become a regular 5.0 occurrence. “Smaller 50,000-60,000t cargoes are more popular and the terminal 4.0 does not always have the opportunity to stockpile larger quantities of diesel for 3.0 export,” a source familiar with operations at the outlet says. But the loading is significant considering the planned 10mn t/yr capacity 2.0 addition to the 15mn t/yr Sever diesel pipeline by 2018. Expansion to 25mn t/yr 1.0 will enable Transneft to divert more diesel to its pipeline system from ports in 0.0 Apr Jul Oct Jan Apr the Baltic states, in particular from the pipeline to the Latvian port of Ventspils. -

Advanced Biofuels Based on Fischer–Tropsch Synthesis for Applications in Gasoline Engines
materials Article Advanced Biofuels Based on Fischer–Tropsch Synthesis for Applications in Gasoline Engines Jiˇrí Hájek 1,2, Vladimír Hönig 1,* , Michal Obergruber 1 , Jan Jenˇcík 1,2 , Aleš Vráblík 2 , Radek Cernˇ ý 2 , Martin Pšeniˇcka 2 and Tomáš Herink 2 1 Department of Chemistry, Faculty of Agrobiology, Food and Natural Resources, Czech University of Life Sciences Prague, Kamýcká 129, 165 00 Prague, Czech Republic; [email protected] (J.H.); [email protected] (M.O.); [email protected] (J.J.) 2 ORLEN UniCRE a.s., 436 01 Litvínov, Czech Republic; [email protected] (A.V.); [email protected] (R.C.);ˇ [email protected] (M.P.); [email protected] (T.H.) * Correspondence: [email protected] Abstract: The aim of the article is to determine the properties of fuel mixtures of Fischer–Tropsch naphtha fraction with traditional gasoline (petrol) to be able to integrate the production of advanced alternative fuel based on Fischer–Tropsch synthesis into existing fuel markets. The density, octane number, vapor pressure, cloud point, water content, sulphur content, refractive index, ASTM color, heat of combustion, and fuel composition were measured using the gas chromatography method PIONA. It was found that fuel properties of Fischer–Tropsch naphtha fraction is not much comparable to conventional gasoline (petrol) due to the high n-alkane content. This research work recommends the creation of a low-percentage mixture of 3 vol.% of FT naphtha fraction with traditional gasoline to minimize negative effects—similar to the current legislative limit of 5 vol.% of bioethanol in E5 Citation: Hájek, J.; Hönig, V.; gasoline. -

Specifications Guide Europe and Africa Refined Oil Products Latest Update: September 2021
Specifications Guide Europe And Africa Refined Oil Products Latest update: September 2021 Definitions of the trading locations for which Platts publishes daily indexes or assessments 2 LPG 4 Gasoline 7 Naphtha 9 Jet fuel 11 ULSD 13 Gasoil 16 Fuel oil 18 Feedstocks 23 Revision history 26 www.spglobal.com/platts Specifications Guide Europe And Africa Refined Oil Products: September 2021 DEFINITIONS OF THE TRADING LOCATIONS FOR WHICH PLATTS PUBLISHES DAILY INDEXES OR ASSESSMENTS The following specifications guide contains the primary specifications and methodologies for Platts refined oil products assessments throughout Europe and Africa. All the assessments listed here employ Platts Assessments Methodology, as published at https://www.spglobal.com/platts/plattscontent/_assets/_files/en/our-methodology/methodology-specifications/platts-assessments-methodology-guide.pdf. These guides are designed to give Platts subscribers as much information as possible about a wide range of methodology and specification questions. This guide is current at the time of publication. Platts may issue further updates and enhancements to this guide and will announce these to subscribers through its usual publications of record. Such updates will be included in the next version of this guide. Platts editorial staff and managers are available to provide guidance when assessment issues require clarification. Unnamed ship Seller has the responsibility to declare its Shipping considerations Northwest European and FOB Mediterranean oil product cargo commitment to meet either the vetting Bids: For the cargo assessment processes bids may be assessments reflect market activity where the seller nominates requirement of any buyer or conversely to expressed with a specific location. Bids with excessive the loading terminal 7 calendar days ahead of the first day of the declare up front how many ship vettings the limitations – whether expressed or implied – may be deemed 5-day laycan. -

5.1 Petroleum Refining1
5.1 Petroleum Refining1 5.1.1 General Description The petroleum refining industry converts crude oil into more than 2500 refined products, including liquefied petroleum gas, gasoline, kerosene, aviation fuel, diesel fuel, fuel oils, lubricating oils, and feedstocks for the petrochemical industry. Petroleum refinery activities start with receipt of crude for storage at the refinery, include all petroleum handling and refining operations and terminate with storage preparatory to shipping the refined products from the refinery. The petroleum refining industry employs a wide variety of processes. A refinery's processing flow scheme is largely determined by the composition of the crude oil feedstock and the chosen slate of petroleum products. The example refinery flow scheme presented in Figure 5.1-1 shows the general processing arrangement used by refineries in the United States for major refinery processes. The arrangement of these processes will vary among refineries, and few, if any, employ all of these processes. Petroleum refining processes having direct emission sources are presented on the figure in bold-line boxes. Listed below are 5 categories of general refinery processes and associated operations: 1. Separation processes a. Atmospheric distillation b. Vacuum distillation c. Light ends recovery (gas processing) 2. Petroleum conversion processes a. Cracking (thermal and catalytic) b. Reforming c. Alkylation d. Polymerization e. Isomerization f. Coking g. Visbreaking 3.Petroleum treating processes a. Hydrodesulfurization b. Hydrotreating c. Chemical sweetening d. Acid gas removal e. Deasphalting 4.Feedstock and product handling a. Storage b. Blending c. Loading d. Unloading 5.Auxiliary facilities a. Boilers b. Waste water treatment c. Hydrogen production d. -

Conversion Factors
CONVERSION FACTORS The data which have been supplied by the countries The conversion of fuelwood (solid volume, 9.135 in original units are converted to the common unit, GJ/m3) is based on an average 20-30% moisture content. terajoules (TJ), by using standard conversion factors or, in When data on the transformation of fuelwood into charcoal the case of solids, specific factors, where available, as are incomplete, estimations assume that it requires 6 cubic indicated below. metres of fuelwood to produce 1 metric ton of charcoal, equivalent to an approximately 53% transformation Specific factors are displayed for production and for efficiency on an energy basis imports. The ones for production are also used for exports and stock changes. All the other flows use a conversion For users wishing to convert the data from terajoules factor which is a weighted average between both. Where (TJ) to other common units: one thousand tons of coal no specific factor is listed for a particular country, the equivalent (1,000 TCE) has traditionally been defined as standard factor is used to convert all data. comprising 29.3076 TJ, and one thousand tons of oil equivalent (1,000 TOE) as 42.6216 TJ. One calorie Standard conversion factors for liquid fuels are corresponds to 4.1868 joules and is the amount of energy determined on the basis of the net calorific value for each which will raise the temperature of one gramme of water product. by one degree Celsius. xxiii STANDARD NET CALORIFIC VALUES (Terajoules per thousand metric tons, unless otherwise stated) -

Material Safety Data Sheet Naphtha
Material Safety Data Sheet Naphtha NFPA: Flammability HMIS III: Reactivity HEALTH 1 3 FLAMMABILITY 3 1 0 Health PHYSICAL 0 0 = Insignificant, 1 = Slight, 2 = Moderate, Specific Hazard 3 = High, 4 = Extreme SECTION 1. PRODUCT AND COMPANY IDENTIFICATION Product name : Naphtha Synonyms : Light Naphtha, Japan Open Spec Bonded Naphtha, SNG Naphtha, Light Cat Naphtha, Sweet Virgin Naphtha (SVN), Debutanized Naphtha, Atmospheric Naphtha (DAN), HCU Light Naphtha, Light CR Gasoline, Full Range Cracked Naphtha, Full Range Hydrocracked Naphtha, Full Range Reformed Naphtha, Light Chemical Treated Naphtha, Light Cracked Naphtha, Light Hydrocracked Naphtha, Light Hydrotreated Naphtha, Aviation Alkylate Naphtha, 888100004450 MSDS Number : 888100004450 Version : 2.12 Product Use Description : Fuel Component, Refinery Intermediate Stream Company : For: Tesoro Refining & Marketing Co. 19100 Ridgewood Parkway, San Antonio, TX 78259 Tesoro Call Center : (877) 783-7676 Chemtrec : (800) 424-9300 (Emergency Contact) SECTION 2. HAZARDS IDENTIFICATION Emergency Overview Regulatory status : This material is considered hazardous by the Occupational Safety and Health Administration (OSHA) Hazard Communication Standard (29 CFR 1910.1200). Signal Word : DANGER Hazard Summary : Extremely flammable. Irritating to eyes and respiratory system. Affects central nervous system. Harmful or fatal if swallowed. Aspiration Hazard. Potential Health Effects Eyes : High vapor concentration or contact may cause irritation and discomfort. Skin : Brief contact may cause slight irritation. -
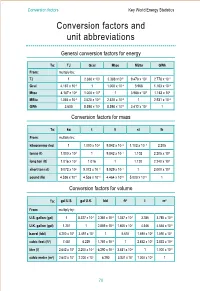
Conversion Factors and Unit Abbreviations
Conversion factors Key World Energy Statistics Conversion factors and unit abbreviations General conversion factors for energy To: TJ Gcal Mtoe MBtu GWh From: multiply by: TJ 1 2.388 x 102 2.388 x10-5 9.478 x 102 2.778 x 10-1 Gcal 4.187 x 10-3 1 1.000 x 10-7 3.968 1.163 x 10-3 Mtoe 4.187 x 104 1.000 x 107 1 3.968 x 107 1.163 x 104 MBtu 1.055 x 10-3 2.520 x 10-1 2.520 x 10-8 1 2.931 x 10-4 GWh 3.600 8.598 x 102 8.598 x 10-5 3.412 x 103 1 Conversion factors for mass To: kg t lt st lb From: multiply by: kilogramme (kg) 1 1.000 x 10-3 9.842 x 10–4 1.102 x 10–3 2.205 tonne (t) 1.000 x 103 1 9.842 x 10–1 1.102 2.205 x 103 long ton (lt) 1.016 x 103 1.016 1 1.120 2.240 x 103 short ton (st) 9.072 x 102 9.072 x 10–1 8.929 x 10–1 1 2.000 x 103 pound (lb) 4.536 x 10–1 4.536 x 10–4 4.464 x 10–4 5.000 x 10–4 1 Conversion factors for volume To: gal U.S. gal U.K. bbl ft3 l m3 From: multiply by: U.S. gallon (gal) 1 8.327 x 10-1 2.381 x 10-2 1.337 x 10-1 3.785 3.785 x 10-3 U.K. -

Changing Global Petroleum Product Trade Flows
Changing Global Petroleum Product Trade Flows 2014 EIA Energy Conference Washington, DC Antoine Halff July 14, 2014 © OECD/IEA 2014 © OECD/IEA 2014 Crude trade shifts further east Crude Exports in 2019 and Growth in 2013-19 for Key Trade Routes1 (million barrels per day) 0.5 (-0.0) OECD 3.6 Europe (-0.5) OECD 0.2 Asia 0.3 1.3 (+0.6) Oceania3 (0) 2.0 (0.2) OECD 2 (-0.6) 1.6 0.7 4.1 Americas (-0.2) (+0.4) (-0.6) China 0.1 (-0.7) 3.1 Other Asia 1.2 (0.3) 1.0 (+0.1) -0.6 2.2 5.2 1.8 (+0.8) (+0.3) (-0.6) 1.1 (+0.3) Red number in brackets denotes growth in period 2013-19 1.2 1Excludes Intra-Regional Trade (+0.6) 2 Includes Chile 3 Includes Israel Asia imports increase by 2.6 mb/d to 22.1 mb, or 65% of the international crude market © OECD/IEA 2014 Refinery capacity expansions continue… Crude Distillation Expansions 2.0 CDU Expansions 2013-2019 by Region 1.5 1.0 mb/d 0.5 0.0 2013 2014 2015 2016 2017 2018 2019 -0.5 OECD North America OECD Europe OECD Pacific FSU China Other Asia Latin America Middle East Africa 95% of new capacity comes from the non-OECD, of which Asia accounts for half © OECD/IEA 2014 …but plans are getting scaled back in the face of rising over-capacity Revisions to capacity expansion plans since 2013 MTOMR 1.5 Chinese CDU expansions vs . -

Neste Oil Template
A different kind of refining company – World’s leading supplier of renewable diesel Dr. Lars Peter Lindfors, SVP Technology 11th Annual World Congress on Industrial Biotechnology May 2014 Neste Oil in brief A refining and Refining capacity: 15 million t/a of marketing company Net sales: € 17.5 petroleum products focused on billion (2013) premium-quality and 2 million t/a of traffic fuels renewable diesel Operations in 15 Listed on the Largest owner: countries; employs Helsinki Stock Finnish State approx. 5,000 Exchange (50.1%) people 2 Being innovative drives our leadership position 1,000 Creating Expanding Several new professionals unique raw material products in R&D and technological base since 2008 engineering innovations 3 NEXBTL renewable fuels – fully compatible with fossil diesel Renewable diesel Biodiesel Fischer-Tropsch Fossil diesel (HVO) (FAME / RME) (BTL) e.g. NEXBTL Flexible mix of raw Vegetable oils & Crude oil materials Raw material animal fats (mainly Biomass (mineral oil) (vegetable oils rapeseed oil) & waste fats) Gasification & Technology Esterification Traditional refining Hydrotreating Fischer-Tropsch Bio-based Bio-based Ester-based, Hydrocarbon hydrocarbon hydrocarbon End product conventional (gasoline, jet fuel, (renewable diesel, (renewable gasoline, biodiesel diesel) jet fuel, bionaphta, jet fuel, diesel) biopropane) O Chemical C H II n 2n+2 C H C H composition + aromatics n 2n+2 n 2n+2 H3C-O-C-R FAME = Fatty Acid Methyl Ester, conventional biodiesel HVO = Hydrotreated Vegetable Oil, advanced biofuel i.e. renewable