Transcriptional Silencing of Long Noncoding RNA GNG12-AS1 Uncouples Its Transcriptional and Product-Related Functions.” Nature Communications 7 (1): 10406
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Original Article Upregulation of HOXA13 As a Potential Tumorigenesis and Progression Promoter of LUSC Based on Qrt-PCR and Bioinformatics
Int J Clin Exp Pathol 2017;10(10):10650-10665 www.ijcep.com /ISSN:1936-2625/IJCEP0065149 Original Article Upregulation of HOXA13 as a potential tumorigenesis and progression promoter of LUSC based on qRT-PCR and bioinformatics Rui Zhang1*, Yun Deng1*, Yu Zhang1, Gao-Qiang Zhai1, Rong-Quan He2, Xiao-Hua Hu2, Dan-Ming Wei1, Zhen-Bo Feng1, Gang Chen1 Departments of 1Pathology, 2Medical Oncology, First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi Zhuang Autonomous Region, China. *Equal contributors. Received September 7, 2017; Accepted September 29, 2017; Epub October 1, 2017; Published October 15, 2017 Abstract: In this study, we investigated the levels of homeobox A13 (HOXA13) and the mechanisms underlying the co-expressed genes of HOXA13 in lung squamous cancer (LUSC), the signaling pathways in which the co-ex- pressed genes of HOXA13 are involved and their functional roles in LUSC. The clinical significance of 23 paired LUSC tissues and adjacent non-tumor tissues were gathered. HOXA13 levels in LUSC were detected by quantita- tive real-time polymerase chain reaction (qRT-PCR). HOXA13 levels in LUSC from The Cancer Genome Atlas (TCGA) and Oncomine were analyzed. We performed receiver operator characteristic (ROC) curves of various clinicopath- ological features of LUSC. Co-expressed of HOXA13 were collected from MEM, cBioPortal and GEPIA. The func- tions and pathways of the most reliable overlapped genes were achieved from the Gene Otology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) databases, respectively. The protein-protein interaction (PPI) net- works were mapped using STRING. HOXA13 in LUSC were markedly upregulated compared with those in the non- cancerous controls as demonstrated by qRT-PCR (LUSC: 0.330±0.360; CONTROLS: 0.155±0.142; P=0.021). -

DIRAS3 Antibody Catalog # ASC11908
10320 Camino Santa Fe, Suite G San Diego, CA 92121 Tel: 858.875.1900 Fax: 858.622.0609 DIRAS3 Antibody Catalog # ASC11908 Specification DIRAS3 Antibody - Product Information Application WB Primary Accession O95661 Other Accession NP_004666, 4757772 Reactivity Human Host Rabbit Clonality Polyclonal Isotype IgG Calculated MW Predicted: 25 kDa Observed: 24 kDa KDa Application Notes DIRAS3 antibody can be used for detection of DIRAS3 by Western blot at 1 Western blot analysis of DIRAS3 in human - 2 µg/ml. testis tissue lysate with DIRAS3 antibody at 1 µg/ml. DIRAS3 Antibody - Additional Information DIRAS3 Antibody - Background Gene ID 9077 Target/Specificity DIRAS3 is a member of the ras superfamily, DIRAS3; DIRAS3 antibody is human specific. and is expressed in normal ovarian and breast epithelial cells, but not in ovarian and breast Reconstitution & Storage cancers. It is an imprinted gene, with DIRAS3 antibody can be stored at 4℃ for mono-allelic expression of the paternal allele, three months and -20℃, stable for up to which is associated with growth suppression one year. and down-regulation of cyclin D1 promoter Precautions activity and induction of p21 (WAF/CIP1). Thus, DIRAS3 Antibody is for research use only this gene appears to be a putative tumor and not for use in diagnostic or therapeutic suppressor gene whose function is abrogated procedures. in ovarian and breast cancers (1). DIRAS3 has been shown to induce autophagy in human ovarian cancer cells by blocking PI3K signaling, inhibiting the mammalian target of rapamycin DIRAS3 Antibody - Protein Information (TOR), upregulating ATG4, and colocalizing with LC3 in autophagosomes (2). DIRAS also Name DIRAS3 interacts with C-RAF and downregulates mitogen-activated protein kinase kinases Synonyms ARHI, NOEY2, RHOI (MEK) to restrict cell migration (3). -

Supplementary Information Method CLEAR-CLIP. Mouse Keratinocytes
Supplementary Information Method CLEAR-CLIP. Mouse keratinocytes of the designated genotype were maintained in E-low calcium medium. Inducible cells were treated with 3 ug/ml final concentration doxycycline for 24 hours before performing CLEAR-CLIP. One 15cm dish of confluent cells was used per sample. Cells were washed once with cold PBS. 10mls of cold PBS was then added and cells were irradiated with 300mJ/cm2 UVC (254nM wavelength). Cells were then scraped from the plates in cold PBS and pelleted by centrifugation at 1,000g for 2 minutes. Pellets were frozen at -80oC until needed. Cells were then lysed on ice with occasional vortexing in 1ml of lysis buffer (50mM Tris-HCl pH 7.4, 100mM NaCl, 1mM MgCl2, 0.1 mM CaCl2, 1% NP-40, 0.5% Sodium Deoxycholate, 0.1% SDS) containing 1X protease inhibitors (Roche #88665) and RNaseOUT (Invitrogen #10777019) at 4ul/ml final concentration. Next, TurboDNase (Invitrogen #AM2238, 10U), RNase A (0.13ug) and RNase T1 (0.13U) were added and samples were incubated at 37oC for 5 minutes with occasional mixing. Samples were immediately placed on ice and then centrifuged at 16,160g at 4oC for 20 minutes to clear lysate. 25ul of Protein-G Dynabeads (Invitrogen #10004D) were used per IP. Dynabeads were pre-washed with lysis buffer and pre- incubated with 3ul of Wako Anti-Mouse-Ago2 (2D4) antibody. The dynabead/antibody mixture was added to the lysate and rocked for 2 hours at 4oC. All steps after the IP were done on bead until samples were loaded into the polyacrylamide gel. -

DIRAS3 Antibody Cat
DIRAS3 Antibody Cat. No.: 8139 DIRAS3 Antibody Specifications HOST SPECIES: Rabbit SPECIES REACTIVITY: Human DIRAS3 antibody was raised against an 18 amino acid peptide near the carboxy terminus of human DIRAS3. IMMUNOGEN: The immunogen is located within the last 50 amino acids of DIRAS3. TESTED APPLICATIONS: ELISA, WB DIRAS3 antibody can be used for detection of DIRAS3 by Western blot at 1 - 2 μg/ml. APPLICATIONS: Antibody validated: Western Blot in human samples. All other applications and species not yet tested. SPECIFICITY: DIRAS3 antibody is human specific. POSITIVE CONTROL: 1) Cat. No. 1313 - Human Testis Tissue Lysate Predicted: 25 kDa PREDICTED MOLECULAR WEIGHT: Observed: 24 kDa Properties September 26, 2021 1 https://www.prosci-inc.com/diras3-antibody-8139.html PURIFICATION: DIRAS3 antibody is affinity chromatography purified via peptide column. CLONALITY: Polyclonal ISOTYPE: IgG CONJUGATE: Unconjugated PHYSICAL STATE: Liquid BUFFER: DIRAS3 antibody is supplied in PBS containing 0.02% sodium azide. CONCENTRATION: 1 mg/mL DIRAS3 antibody can be stored at 4˚C for three months and -20˚C, stable for up to one STORAGE CONDITIONS: year. Additional Info OFFICIAL SYMBOL: DIRAS3 ALTERNATE NAMES: DIRAS family GTP-binding RAS-like 3, ARHI, NOEY2 ACCESSION NO.: NP_004666 PROTEIN GI NO.: 4757772 GENE ID: 9077 USER NOTE: Optimal dilutions for each application to be determined by the researcher. Background and References DIRAS3 is a member of the ras superfamily, and is expressed in normal ovarian and breast epithelial cells, but not in ovarian and breast cancers. It is an imprinted gene, with mono-allelic expression of the paternal allele, which is associated with growth suppression and down-regulation of cyclin D1 promoter activity and induction of p21 (WAF/CIP1). -

Palmitic Acid Effects on Hypothalamic Neurons
bioRxiv preprint doi: https://doi.org/10.1101/2021.08.03.454666; this version posted August 4, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Running title: Oleic and palmitic acid effects on hypothalamic neurons Concentration-dependent change in hypothalamic neuronal transcriptome by the dietary fatty acids: oleic and palmitic acids Fabiola Pacheco Valencia1^, Amanda F. Marino1^, Christos Noutsos1, Kinning Poon1* 1Department of Biological Sciences, SUNY Old Westbury, Old Westbury NY, United States ^Authors contributed equally to this work *Corresponding Author: Kinning Poon 223 Store Hill Rd Old Westbury, NY 11568, USA 1-516-876-2735 [email protected] bioRxiv preprint doi: https://doi.org/10.1101/2021.08.03.454666; this version posted August 4, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Abstract Prenatal high-fat diet exposure increases hypothalamic neurogenesis events in embryos and programs offspring to be obesity-prone. The molecular mechanism involved in these dietary effects of neurogenesis are unknown. This study investigated the effects of oleic and palmitic acids, which are abundant in a high-fat diet, on the hypothalamic neuronal transcriptome and how these changes impact neurogenesis events. The results show differential effects of low and high concentrations of oleic or palmitic acid treatment on differential gene transcription. -

G Protein-Coupled Receptors
G PROTEIN-COUPLED RECEPTORS Overview:- The completion of the Human Genome Project allowed the identification of a large family of proteins with a common motif of seven groups of 20-24 hydrophobic amino acids arranged as α-helices. Approximately 800 of these seven transmembrane (7TM) receptors have been identified of which over 300 are non-olfactory receptors (see Frederikson et al., 2003; Lagerstrom and Schioth, 2008). Subdivision on the basis of sequence homology allows the definition of rhodopsin, secretin, adhesion, glutamate and Frizzled receptor families. NC-IUPHAR recognizes Classes A, B, and C, which equate to the rhodopsin, secretin, and glutamate receptor families. The nomenclature of 7TM receptors is commonly used interchangeably with G protein-coupled receptors (GPCR), although the former nomenclature recognises signalling of 7TM receptors through pathways not involving G proteins. For example, adiponectin and membrane progestin receptors have some sequence homology to 7TM receptors but signal independently of G-proteins and appear to reside in membranes in an inverted fashion compared to conventional GPCR. Additionally, the NPR-C natriuretic peptide receptor has a single transmembrane domain structure, but appears to couple to G proteins to generate cellular responses. The 300+ non-olfactory GPCR are the targets for the majority of drugs in clinical usage (Overington et al., 2006), although only a minority of these receptors are exploited therapeutically. Signalling through GPCR is enacted by the activation of heterotrimeric GTP-binding proteins (G proteins), made up of α, β and γ subunits, where the α and βγ subunits are responsible for signalling. The α subunit (tabulated below) allows definition of one series of signalling cascades and allows grouping of GPCRs to suggest common cellular, tissue and behavioural responses. -

Supplementary Table 1
Supplementary Table 1. Large-scale quantitative phosphoproteomic profiling was performed on paired vehicle- and hormone-treated mTAL-enriched suspensions (n=3). A total of 654 unique phosphopeptides corresponding to 374 unique phosphoproteins were identified. The peptide sequence, phosphorylation site(s), and the corresponding protein name, gene symbol, and RefSeq Accession number are reported for each phosphopeptide identified in any one of three experimental pairs. For those 414 phosphopeptides that could be quantified in all three experimental pairs, the mean Hormone:Vehicle abundance ratio and corresponding standard error are also reported. Peptide Sequence column: * = phosphorylated residue Site(s) column: ^ = ambiguously assigned phosphorylation site Log2(H/V) Mean and SE columns: H = hormone-treated, V = vehicle-treated, n/a = peptide not observable in all 3 experimental pairs Sig. column: * = significantly changed Log 2(H/V), p<0.05 Log (H/V) Log (H/V) # Gene Symbol Protein Name Refseq Accession Peptide Sequence Site(s) 2 2 Sig. Mean SE 1 Aak1 AP2-associated protein kinase 1 NP_001166921 VGSLT*PPSS*PK T622^, S626^ 0.24 0.95 PREDICTED: ATP-binding cassette, sub-family A 2 Abca12 (ABC1), member 12 XP_237242 GLVQVLS*FFSQVQQQR S251^ 1.24 2.13 3 Abcc10 multidrug resistance-associated protein 7 NP_001101671 LMT*ELLS*GIRVLK T464, S468 -2.68 2.48 4 Abcf1 ATP-binding cassette sub-family F member 1 NP_001103353 QLSVPAS*DEEDEVPVPVPR S109 n/a n/a 5 Ablim1 actin-binding LIM protein 1 NP_001037859 PGSSIPGS*PGHTIYAK S51 -3.55 1.81 6 Ablim1 actin-binding -

Multi-Functionality of Proteins Involved in GPCR and G Protein Signaling: Making Sense of Structure–Function Continuum with In
Cellular and Molecular Life Sciences (2019) 76:4461–4492 https://doi.org/10.1007/s00018-019-03276-1 Cellular andMolecular Life Sciences REVIEW Multi‑functionality of proteins involved in GPCR and G protein signaling: making sense of structure–function continuum with intrinsic disorder‑based proteoforms Alexander V. Fonin1 · April L. Darling2 · Irina M. Kuznetsova1 · Konstantin K. Turoverov1,3 · Vladimir N. Uversky2,4 Received: 5 August 2019 / Revised: 5 August 2019 / Accepted: 12 August 2019 / Published online: 19 August 2019 © Springer Nature Switzerland AG 2019 Abstract GPCR–G protein signaling system recognizes a multitude of extracellular ligands and triggers a variety of intracellular signal- ing cascades in response. In humans, this system includes more than 800 various GPCRs and a large set of heterotrimeric G proteins. Complexity of this system goes far beyond a multitude of pair-wise ligand–GPCR and GPCR–G protein interactions. In fact, one GPCR can recognize more than one extracellular signal and interact with more than one G protein. Furthermore, one ligand can activate more than one GPCR, and multiple GPCRs can couple to the same G protein. This defnes an intricate multifunctionality of this important signaling system. Here, we show that the multifunctionality of GPCR–G protein system represents an illustrative example of the protein structure–function continuum, where structures of the involved proteins represent a complex mosaic of diferently folded regions (foldons, non-foldons, unfoldons, semi-foldons, and inducible foldons). The functionality of resulting highly dynamic conformational ensembles is fne-tuned by various post-translational modifcations and alternative splicing, and such ensembles can undergo dramatic changes at interaction with their specifc partners. -

GNG12-AS1 Uncouples Its Transcriptional and Product-Related Functions
ARTICLE Received 4 Sep 2015 | Accepted 8 Dec 2015 | Published 2 Feb 2016 DOI: 10.1038/ncomms10406 OPEN Transcriptional silencing of long noncoding RNA GNG12-AS1 uncouples its transcriptional and product-related functions Lovorka Stojic1, Malwina Niemczyk1, Arturo Orjalo2,w, Yoko Ito1, Anna Elisabeth Maria Ruijter1, Santiago Uribe-Lewis1, Nimesh Joseph1, Stephen Weston3, Suraj Menon1, Duncan T. Odom1, John Rinn4, Fanni Gergely1 & Adele Murrell1,3 Long noncoding RNAs (lncRNAs) regulate gene expression via their RNA product or through transcriptional interference, yet a strategy to differentiate these two processes is lacking. To address this, we used multiple small interfering RNAs (siRNAs) to silence GNG12-AS1,a nuclear lncRNA transcribed in an antisense orientation to the tumour-suppressor DIRAS3. Here we show that while most siRNAs silence GNG12-AS1 post-transcriptionally, siRNA complementary to exon 1 of GNG12-AS1 suppresses its transcription by recruiting Argonaute 2 and inhibiting RNA polymerase II binding. Transcriptional, but not post-transcriptional, silencing of GNG12-AS1 causes concomitant upregulation of DIRAS3, indicating a function in transcriptional interference. This change in DIRAS3 expression is sufficient to impair cell cycle progression. In addition, the reduction in GNG12-AS1 transcripts alters MET signalling and cell migration, but these are independent of DIRAS3. Thus, differential siRNA targeting of a lncRNA allows dissection of the functions related to the process and products of its transcription. 1 Cancer Research UK Cambridge Institute, University of Cambridge, Li Ka Shing Centre, Robinson Way, Cambridge CB2 0RE, UK. 2 Biosearch Technologies Inc., 2199S. McDowell Boulevard, Petaluma, California 94954, USA. 3 Centre for Regenerative Medicine, Department of Biology and Biochemistry, University of Bath, Claverton Down, Bath BA2 7AY, UK. -

ARHI (DIRAS3), an Imprinted Tumour Suppressor Gene, Binds to Importins and Blocks Nuclear Import of Cargo Proteins
Biosci. Rep. (2010) / 30 / 159–168 (Printed in Great Britain) / doi 10.1042/BSR20090008 ARHI (DIRAS3), an imprinted tumour suppressor gene, binds to importins and blocks nuclear import of cargo proteins Shaoyi HUANG*, In Soon CHANG†, Wenbo LIN§, Wenduo YE§, Robert Z. LUO*, Zhen LU*, Yiling LU‡, Ke ZHANG†, Warren S.-L. LIAO*, Tao TAO§, Robert C. BAST, Jr*, Xiaomin CHEN† and Yinhua YU*1 *Department of Experimental Therapeutics, The University of Texas, M.D. Anderson Cancer Center, Houston, TX 77030, U.S.A., †Department of Biochemistry and Molecular Biology, The University of Texas, M.D. Anderson Cancer Center, Houston, TX 77030, U.S.A., ‡Department of Systems Biology, The University of Texas, M.D. Anderson Cancer Center, Houston, Texas 77030, U.S.A., §School of Life Sciences, Xiamen University, Xiamen, Fujian 361005, People’s Republic of China, and Obstetrics and Gynecology Hospital of Fudan University, Shanghai 200011, People’s Republic of China ' $ Synopsis ARHI (aplasia Ras homologue member I; also known as DIRAS3) is an imprinted tumour suppressor gene, the expression of which is lost in the majority of breast and ovarian cancers. Unlike its homologues Ras and Rap, ARHI functions as a tumour suppressor. Our previous study showed that ARHI can interact with the transcriptional activator STAT3 (signal transducer and activator of transcription 3) and inhibit its nuclear translocation in human breast- and ovarian-cancer cells. To identify proteins that interact with ARHI in nuclear translocation, in the present study, we performed proteomic analysis and identified several importins that can associate with ARHI. To further explore this novel finding, we purified 10 GST (glutathione transferase)–importin fusion proteins (importins 7, 8, 13, β1, α1, α3, α5, α6, α7 and mutant α1). -

Human ARHI / DIRAS3 Protein (Fc Tag)
Human ARHI / DIRAS3 Protein (Fc Tag) Catalog Number: 14239-H04H General Information SDS-PAGE: Gene Name Synonym: ARHI; DIRAS3; NOEY2; RHOI Protein Construction: A DNA sequence encoding the human DIRAS3 (O95661) (Met1-Lys225) was expressed with the Fc region of mouse IgG1 at the N-terminus. Source: Human Expression Host: HEK293 Cells QC Testing Purity: > 85 % as determined by SDS-PAGE Endotoxin: Protein Description < 1.0 EU per μg of the protein as determined by the LAL method ARHI, also known as DIRAS3, belongs to the small GTPase superfamily, Stability: Di-Ras family. ARHI gene is a novel tumor suppressor gene located on chromosome 1p31. Downregulation of ARHI expression has been detected Samples are stable for up to twelve months from date of receipt at -70 ℃ in many types of cancer. ARHI is expressed in normal ovarian and breast epithelial cells but not in ovarian and breast cancers. As a suppressor, Predicted N terminal: Asp ARHI is not only an important factor in the pathogenesis of gastric cancer, Molecular Mass: but also a potential factor for tumor aggravation. ARHI expression in gastric cancer can be employed to indicate favorable prognosis for the disease. The recombinant human DIRAS3/mFc comprises 461 amino acids and has a predicted molecular mass of 52.1 kDa. The apparent molecular References mass of the monomer is approximately 62 kDa in SDS-PAGE under 1.Pei XH. et al., 2011, Cell Biol Int. 35 (10): 1019-24. 2.Lin D. et al., 2011, reducing conditions due to glycosylation. J Int Med Res. 39 (5): 1870-5. -
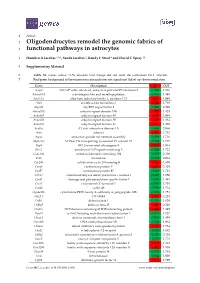
Oligodendrocytes Remodel the Genomic Fabrics of Functional Pathways in Astrocytes
1 Article 2 Oligodendrocytes remodel the genomic fabrics of 3 functional pathways in astrocytes 4 Dumitru A Iacobas 1,2,*, Sanda Iacobas 3, Randy F Stout 4 and David C Spray 2,5 5 Supplementary Material 6 Table S1. Genes whose >1.5x absolute fold-change did not meet the individual CUT criterion. 7 Red/green background of the expression ratio indicates not significant (false) up-/down-regulation. Gene Description X CUT Acap2 ArfGAP with coiled-coil, ankyrin repeat and PH domains 2 -1.540 1.816 Adamts18 a disintegrin-like and metallopeptidase -1.514 1.594 Akr1c12 aldo-keto reductase family 1, member C12 1.866 1.994 Alx3 aristaless-like homeobox 3 1.536 1.769 Alyref2 Aly/REF export factor 2 -1.880 2.208 Ankrd33b ankyrin repeat domain 33B 1.593 1.829 Ankrd45 ankyrin repeat domain 45 1.514 1.984 Ankrd50 ankyrin repeat domain 50 1.628 1.832 Ankrd61 ankyrin repeat domain 61 1.645 1.802 Arid1a AT rich interactive domain 1A -1.668 2.066 Artn artemin 1.524 1.732 Aspm abnormal spindle microtubule assembly -1.693 1.716 Atp6v1e1 ATPase, H+ transporting, lysosomal V1 subunit E1 -1.679 1.777 Bag4 BCL2-associated athanogene 4 1.723 1.914 Birc3 baculoviral IAP repeat-containing 3 -1.588 1.722 Ccdc104 coiled-coil domain containing 104 -1.819 2.130 Ccl2 chemokine -1.699 2.034 Cdc20b cell division cycle 20 homolog B 1.512 1.605 Cenpf centromere protein F 2.041 2.128 Cep97 centrosomal protein 97 -1.641 1.723 COX1 mitochondrially encoded cytochrome c oxidase I -1.607 1.650 Cpsf7 cleavage and polyadenylation specific factor 7 -1.635 1.891 Crct1 cysteine-rich