Molecular Beam Epitaxy in the Lithium-Niobium-Oxygen System
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Theoretical and Experimental Aspects of Current and Future Research on Nbo2 Thin Film Devices
crystals Review Theoretical and Experimental Aspects of Current and Future Research on NbO2 Thin Film Devices Denis Music 1,* , Andreas M. Krause 1 and Pär A. T. Olsson 1,2 1 Department of Materials Science and Applied Mathematics, Malmö University, SE-205 06 Malmö, Sweden; [email protected] (A.M.K.); [email protected] (P.A.T.O.) 2 Division of Mechanics, Lund University, Box 118, SE-221 00 Lund, Sweden * Correspondence: [email protected]; Tel.: +46-40-6658709 Abstract: The present research front of NbO2 based memory, energy generation, and storage thin film devices is reviewed. Sputtering plasmas contain NbO, NbO2, and NbO3 clusters, affecting nucleation and growth of NbO2, often leading to a formation of nanorods and nanoslices. NbO2 (I41/a) undergoes the Mott topological transition at 1081 K to rutile (P42/mnm), yielding changes in the electronic structure, which is primarily utilized in memristors. The Seebeck coefficient is a key physical parameter governing the performance of thermoelectric devices, but its temperature behavior is still controversial. Nonetheless, they perform efficiently above 900 K. There is a great potential to improve NbO2 batteries since the theoretical capacity has not been reached, which may be addressed by future diffusion studies. Thermal management of functional materials, comprising thermal stress, thermal fatigue, and thermal shock, is often overlooked even though it can lead to failure. NbO2 exhibits relatively low thermal expansion and high elastic modulus. The future for NbO2 thin film devices looks promising, but there are issues that need to be tackled, such as dependence of properties on strain and grain size, multiple interfaces with point and extended defects, and interaction with various natural and artificial environments, enabling multifunctional Citation: Music, D.; Krause, A.M.; applications and durable performance. -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -

Interagency Committee on Chemical Management
DECEMBER 14, 2018 INTERAGENCY COMMITTEE ON CHEMICAL MANAGEMENT EXECUTIVE ORDER NO. 13-17 REPORT TO THE GOVERNOR WALKE, PETER Table of Contents Executive Summary ...................................................................................................................... 2 I. Introduction .......................................................................................................................... 3 II. Recommended Statutory Amendments or Regulatory Changes to Existing Recordkeeping and Reporting Requirements that are Required to Facilitate Assessment of Risks to Human Health and the Environment Posed by Chemical Use in the State ............................................................................................................................ 5 III. Summary of Chemical Use in the State Based on Reported Chemical Inventories....... 8 IV. Summary of Identified Risks to Human Health and the Environment from Reported Chemical Inventories ........................................................................................................... 9 V. Summary of any change under Federal Statute or Rule affecting the Regulation of Chemicals in the State ....................................................................................................... 12 VI. Recommended Legislative or Regulatory Action to Reduce Risks to Human Health and the Environment from Regulated and Unregulated Chemicals of Emerging Concern .............................................................................................................................. -

Official Journal of the Patent Office
¯Öê™ëü™ü úÖµÖÖÔ»ÖµÖ úÖ ¿ÖÖÃÖúßµÖ •Ö−ÖÔ»Ö OFFICIAL JOURNAL OF THE PATENT OFFICE (SUPPLEMENT TO THE OFFICIAL JOURNAL DATED 27/03/2009) ×−ÖÖÔ´Ö−Ö ÃÖÓ. 13/2009 ¿ÖãÎú¾ÖÖ¸ ü פü−ÖÖÓú: 27/03/2009 ISSUE NO. 13/2009 FRIDAY DATE: 27/03/2009 ¯Öê™ëü™ü úÖµÖÖÔ»ÖµÖ úÖ ‹ú ¯ÖÏúÖ¿Ö−Ö PUBLICATION OF THE PATENT OFFICE The Patent Office Journal 27/03/2009 16607 CONTENTS SUBJECT PAGE NUMBER JURISDICTION : 16609-16610 PUBLICATION UNDER SECTION 43(2) IN RESPE CT : 16611-16776 OF THE GRANT (DELHI) PUBLICATION UNDER SECTION 43(2) IN RESPEC T : 16777-16779 OF THE GRANT (MUMBAI) PUBLICATION UNDER SECTION 43(2) IN RESPE CT : 16780-16788 OF THE GRANT (CHENNAI) The Patent Office Journal 27/03/2009 16608 THE PATENT OFFICE KOLKATA, 27/03/2009 Address of the Patent Offices/Jurisdictions The following are addresses of all the Patent Offices located at different places having their Territorial Jurisdiction on a Zonal basis as shown below:- 1 Office of the Controller General of Patents, 4 The Patent Office, Designs & Trade Marks, Government of India, Boudhik Sampada Bhavan, Intellectual Property Rights Building, Near Antop Hill Post Office,S.M.Road,Antop Hill, G.S.T. Road, Guindy, Mumbai – 400 037 Chennai – 600 032. Phone: (91)(22) 24123311, Phone: (91)(44) 2250 2081-84 Fax : (91)(22) 24123322 Fax : (91)(44) 2250 2066 E-mail: [email protected] E-mail: [email protected] The States of Andhra Pradesh, Karnataka, Kerala, Tamil Nadu and the Union Territories of Puducherry and Lakshadweep. 2 The Patent Office, Government of India, 5 The Patent Office (Head Office), Boudhik Sampada -

Transition Metal Oxides
. _ ^ ^ „ OCT 14 1975 175118 '-cy NSRDS-NBS 49 Transition Metal Oxides U.S. DEPARTMENT OF COMMERCE/National Bureau of Standards NSRDS Ice dat* Crystal Chemistry, Phase Transition and Related Aspects NATIONAL BUREAU OF STANDARDS The National Bureau of Standards 1 was established by an act of Congress March 3, 1901. The Bureau’s overall goal is to strengthen and advance the Nation’s science and technology and facilitate their effective application for public benefit. To this end, the Bureau conducts research and provides: (1) a basis for the Nation’s physical measurement system, (2) scientific and technological services for industry and government, (3) a technical basis for equity in trade, and (4) technical services to promote public safety. The Bureau consists of the Institute for Basic Standards, the Institute for Materials Research, the Institute for Applied Technology, the Institute for Computer Sciences and Technology, and the Office for Information Programs. THE INSTITUTE FOR BASIC STANDARDS provides the central basis within the United States of a complete and consistent system of physical measurement; coordinates that system with measurement systems of other nations; and furnishes essential services leading to accurate and uniform physical measurements throughout the Nation’s scientific community, industry, and commerce. The Institute consists of a Center for Radiation Research, an Office of Meas- urement Services and the following divisions: Applied Mathematics — Electricity — Mechanics — Heat — Optical Physics — Nuclear Sciences 2 — Applied Radiation 2 — Quantum Electronics 3 — Electromagnetics 3 — Time 3 3 3 and Frequency — Laboratory Astrophysics — Cryogenics . THE INSTITUTE FOR MATERIALS RESEARCH conducts materials research leading to improved methods of measurement, standards, and data on the properties of well-characterized materials needed by industry, commerce, educational institutions, and Government; provides advisory and research services to other Government agencies; and develops, produces, and distributes standard reference materials. -

Open Thesis.Pdf
The Pennsylvania State University The Graduate School Department of Chemistry ALKYNYLIODONIUM SALTS IN ORGANIC SYNTHESIS. APPLICATION TOWARDS THE SYNTHESIS OF THE CORE OF (±)-HALICHLORNIE. and ATTEMPTS TOWARDS THE SYNTHESIS OF KINAMYCIN F. A Thesis in Chemistry by Angela Lyn Perkins © 2005 Angela Lyn Perkins Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy August 2005 The thesis of Angela Lyn Perkins was reviewed and approved* by the following: Ken S. Feldman Professor of Chemistry Thesis Advisor Chair of Committee Blake R. Peterson Associate Professor of Chemistry Raymond L. Funk Professor of Chemistry Squire J. Booker Assistant Professor of Biochemistry and Molecular Biology Ayusman Sen Professor of Chemistry Head of the Department of Chemistry *Signatures are on file in the Graduate School iii ABSTRACT Alkynyliodonium salts are synthetically useful intermediates that serve as electrophilic acetylene equivalents due to the electron withdrawing nature of the hypervalent iodine. In one example, reaction of an alkynyliodonium salt with soft nucleophiles via conjugate addition, followed by loss of iodobenzene, generates alkylidenecarbenes. Alkylidenecarbenes are divalent, short lived intermediates capable of participating in a various bond-forming processes depending upon the functionality present within the molecule. Due to the wide variety of possible reactions, alkylidenecarbenes are useful reactive intermediates in natural product synthesis. The application of alkynyliodonium salts to generate alkylidenecarbenes is described in the first part of this thesis. Chapter 1 discusses the formation of alkynyliodonium salts and their application towards the generation of alkylidenecarbenes. The total synthesis of radermachol, a natural product target, was examined utilizing an alkylidenecarbene addition to a double bond as a key step. -

A Solution and Solid State Study of Niobium Complexes University Of
A solution and solid state study of niobium complexes by Leandra Herbst A dissertation submitted to meet the requirements for the degree of Magister Scientiae in the Department of Chemistry Faculty of Natural- and Agricultural Sciences at the University of the Free State Supervisor: Prof. Andreas Roodt Co-Supervisor: Prof. Hendrik G. Visser January 2012 Acknowledgements First and foremost, I thank God Almighty for the countless blessings he has given me. Thank you to Prof. Roodt for all the opportunities, patience and endless enthusiasm for chemistry, inspiring me to learn as much as I can. It is truly an honour to be known as one of your students. To Prof. Deon Visser, thank you for all your guidance and encouragement. Your neverending patience and willingness to give advice is what kept me motivated throughout my studies. To Dr. Linette Bennie, thank you for your guidance with the niobium NMR throughout this project. Thank you to all my colleagues in the Inorganic group for all the laughter and jokes. Thank you for sharing your knowledge and for your patience when having to explain something several times. Every one of you contributed to this study in some way and for that I thank you. To my father, Lucas Herbst, my sister, Shané Herbst, and my grandmother, Nellie Grobler, without your love, support, faith, sacrifices, understanding and continuous encouragement this would not be possible. Financial assistance from the Advanced Metals Initiative (AMI) of the Department of Science and Technology (DST) of South Africa, through The New Metals Development Network (NMDN) coordinated by the South African Nuclear Energy Corporation Limited (Necsa), and the University of the Free State are gratefully acknowledged. -

The Effect of Stress on the Low-Temperature Oxidation of Niobium Lawrence John Weirick Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1969 The effect of stress on the low-temperature oxidation of niobium Lawrence John Weirick Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Metallurgy Commons Recommended Citation Weirick, Lawrence John, "The effect of stress on the low-temperature oxidation of niobium " (1969). Retrospective Theses and Dissertations. 3617. https://lib.dr.iastate.edu/rtd/3617 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. This dissertation has been microfihned exactly as received 69-20,683 WEIRICK, Lawrence John, 1941- THE EFFECT OF STRESS ON THE LOW- TEMPERATURE OXIDATION OF NIOBIUM. Iowa State University, Ph.D., 1969 Engineering, metallurgy University Microfilms, Inc., Ann Arbor, Michigan THE EFFECT OF STRESS ON THE LOW-TEMPERATURE OXIDATION OF NIOBIUM by Lawrence John Weirick A Dissertation Submitted to the Graduate Faculty in Partial Fulfillment of The Requirements for the Degree of DOCTOR OF PHILOSOPHY Major Subject: Metallurgy Approved: Signature was redacted for privacy. In Charge of Major Work Signature was redacted for privacy. Signature was redacted for privacy. uate -

Pp-03-25-New Dots.Qxd 10/23/02 2:41 PM Page 611
pp-03-25-new dots.qxd 10/23/02 2:41 PM Page 611 NICKEL CARBONATE 611 NICKEL CARBONATE [3333-67-3] Formula: NiCO3; MW 118.72 Two basic carbonates are known. They are 2NiCO3•3Ni(OH)2•4H2O [29863- 10-3], and NiCO3•2Ni(OH)2 [12607-70-4], MW 304.17. The second form occurs in nature as a tetrahydrate, mineral, zaratite. Commercial nickel car- bonate is usually the basic salt, 2NiCO3•3Ni(OH)2•4H2O. Uses Nickel carbonate is used to prepare nickel catalysts and several specialty compounds of nickel. It also is used as a neutralizing agent in nickel plating solutions. Other applications are in coloring glass and in the manufacture of ceramic pigments. Physical Properties NiCO3: Light green rhombohedral crystals; decomposes on heating; practi- cally insoluble in water, 93 mg/L at 25°C; dissolves in acids. 2NiCO3•3Ni(OH)2•4H2O: Light green crystals or brown powder; decom- poses on heating; insoluble in water; decomposes in hot water; soluble in acids and in ammonium salts solutions. Zaratite: Emerald greed cubic crystals; density 2.6 g/cm3; insoluble in water; soluble in ammonia and dilute acids. Thermochemical Properties ∆Ηƒ° (NiCO3) –140.6 kcal/mol Preparation Anhydrous nickel carbonate is produced as a precipitate when calcium car- bonate is heated with a solution of nickel chloride in a sealed tube at 150°C. Alternatively, treating nickel powder with ammonia and carbon dioxide fol- lowed by boiling off ammonia yields pure carbonate. When sodium carbonate is added to a solution of Ni(II) salts, basic nickel carbonate precipitates out in impure form. -

EELS Investigations of Different Niobium Oxide Phases
Microsc. Microanal. 12, 416–423, 2006 / DOI: 10.1017 S1431927606060521 Microscopy AND Microanalysis © MICROSCOPY SOCIETY OF AMERICA 2006 EELS Investigations of Different Niobium Oxide Phases D. Bach,1,* H. Störmer,1 R. Schneider,1 D. Gerthsen,1 and J. Verbeeck2 1Laboratorium für Elektronenmikroskopie, Universität Karlsruhe (TH), D-76128 Karlsruhe, Germany 2Electron Microscopy for Materials Research (EMAT), University of Antwerp, Groenenborgerlaan 171, 2020 Antwerp, Belgium Abstract: Electron energy loss spectra in conjunction with near-edge fine structures of purely stoichiometric niobium monoxide ~NbO! and niobium pentoxide ~Nb2O5! reference materials were recorded. The structures of the niobium oxide reference materials were checked by selected area electron diffraction to ensure a proper assignment of the fine structures. NbO and Nb2O5 show clearly different energy loss near-edge fine structures of the Nb-M4,5 and -M2,3 edges and of the O-K edge, reflecting the specific local environments of the ionized atoms. To distinguish the two oxides in a quantitative manner, the intensities under the Nb-M4,5 as well as Nb-M2,3 edges and the O-K edge were measured and their ratios calculated. k-factors were also derived from these measurements. Key words: niobium monoxide, niobium pentoxide, EELS, ELNES, quantification, stoichiometry INTRODUCTION nanoscale dielectric oxide layers in capacitor structures is essential. Such studies are of importance to understand the mechanisms of leakage currents, which might be due to Niobium and niobium-based compounds have been stud- n-type semiconducting behavior of parts of the dielectric ied for several decades. They find many technological appli- oxide layer as a result of oxygen deficiencies. -

Transition Metal Oxides
. _ ^ ^ „ OCT 14 1975 175118 '-cy NSRDS-NBS 49 Transition Metal Oxides U.S. DEPARTMENT OF COMMERCE/National Bureau of Standards NSRDS Ice dat* Crystal Chemistry, Phase Transition and Related Aspects NATIONAL BUREAU OF STANDARDS The National Bureau of Standards 1 was established by an act of Congress March 3, 1901. The Bureau’s overall goal is to strengthen and advance the Nation’s science and technology and facilitate their effective application for public benefit. To this end, the Bureau conducts research and provides: (1) a basis for the Nation’s physical measurement system, (2) scientific and technological services for industry and government, (3) a technical basis for equity in trade, and (4) technical services to promote public safety. The Bureau consists of the Institute for Basic Standards, the Institute for Materials Research, the Institute for Applied Technology, the Institute for Computer Sciences and Technology, and the Office for Information Programs. THE INSTITUTE FOR BASIC STANDARDS provides the central basis within the United States of a complete and consistent system of physical measurement; coordinates that system with measurement systems of other nations; and furnishes essential services leading to accurate and uniform physical measurements throughout the Nation’s scientific community, industry, and commerce. The Institute consists of a Center for Radiation Research, an Office of Meas- urement Services and the following divisions: Applied Mathematics — Electricity — Mechanics — Heat — Optical Physics — Nuclear Sciences 2 — Applied Radiation 2 — Quantum Electronics 3 — Electromagnetics 3 — Time 3 3 3 and Frequency — Laboratory Astrophysics — Cryogenics . THE INSTITUTE FOR MATERIALS RESEARCH conducts materials research leading to improved methods of measurement, standards, and data on the properties of well-characterized materials needed by industry, commerce, educational institutions, and Government; provides advisory and research services to other Government agencies; and develops, produces, and distributes standard reference materials. -
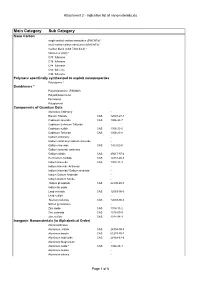
Attachment 2 - Indicative List of Nanomaterials.Xls
Attachment 2 - Indicative list of nanomaterials.xls Main Category Sub Category Nano Carbon single-walled carbon nanotubes (SWCNTs) * multi-walled carbon nanotubes (MWCNTs) * Carbon black (CAS 7440-44-0) * fullerenes (C60) * C70 fullerene C76 fullerene C84 fullerene C90 fullerene C94 fullerene Polymers specifically synthesized to exploit nanoproperties Polystyrene * Dendrimers * Polyamidoamine (PAMAM) Polyethylene imine Ferrocenyl Polyglycerol Components of Quantum Dots Aluminum Antinomy - Barium Titanate CAS 12047-27-7 Cadmium selenide CAS 1306-24-7 Cadmium Selenium Telluride Cadmium sulfide CAS 1306-23-6 Cadmium Telluride CAS 1306-25-8 Gallium Antimony - Gallium antimony/ Gallium arsenide - Gallium Arsenide CAS 1303-00-0 Gallium arsenide antimony Gallium nitride CAS 25617-97-4 Germanium carbide CAS 12334-26-8 Indium Arsenide CAS 1303-11-3 Indium Arsenide Antimony - Indium Arsenide/ Gallium arsenide - Indium Gallium Arsenide - Indium Gallium Nitride - Indium phosphide CAS 22398-80-7 Indium tin oxide - Lead selenide CAS 12069-00-0 Lead sulfide - Mercury telluride CAS 12068-90-5 Silicon germanium - Zinc oxide CAS 1314-13-2 Zinc selenide CAS 1315-09-9 Zinc sulfide CAS 1314-98-3 Inorganic Nanomaterials (in Alphabetical Order) Aluminosilicates - Aluminum nitride CAS 24304-00-5 Aluminum borate CAS 61279-70-7 Aluminum hydroxide CAS 20768-67-6 Aluminum Magnesium - Aluminum oxide * CAS 1344-28-1 Aluminum oxides - Aluminum silicate - Page 1 of 6 Attachment 2 - Indicative list of nanomaterials.xls Main Category Sub Category Aluminum Titanium Oxide