Induction of Beta3-Integrin Gene Expression by Sustained Activation of the Ras-Regulated Raf-MEK-Extracellular Signal- Regulated Kinase Signaling Pathway
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Supplementary Table 1: Adhesion Genes Data Set
Supplementary Table 1: Adhesion genes data set PROBE Entrez Gene ID Celera Gene ID Gene_Symbol Gene_Name 160832 1 hCG201364.3 A1BG alpha-1-B glycoprotein 223658 1 hCG201364.3 A1BG alpha-1-B glycoprotein 212988 102 hCG40040.3 ADAM10 ADAM metallopeptidase domain 10 133411 4185 hCG28232.2 ADAM11 ADAM metallopeptidase domain 11 110695 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 195222 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 165344 8751 hCG20021.3 ADAM15 ADAM metallopeptidase domain 15 (metargidin) 189065 6868 null ADAM17 ADAM metallopeptidase domain 17 (tumor necrosis factor, alpha, converting enzyme) 108119 8728 hCG15398.4 ADAM19 ADAM metallopeptidase domain 19 (meltrin beta) 117763 8748 hCG20675.3 ADAM20 ADAM metallopeptidase domain 20 126448 8747 hCG1785634.2 ADAM21 ADAM metallopeptidase domain 21 208981 8747 hCG1785634.2|hCG2042897 ADAM21 ADAM metallopeptidase domain 21 180903 53616 hCG17212.4 ADAM22 ADAM metallopeptidase domain 22 177272 8745 hCG1811623.1 ADAM23 ADAM metallopeptidase domain 23 102384 10863 hCG1818505.1 ADAM28 ADAM metallopeptidase domain 28 119968 11086 hCG1786734.2 ADAM29 ADAM metallopeptidase domain 29 205542 11085 hCG1997196.1 ADAM30 ADAM metallopeptidase domain 30 148417 80332 hCG39255.4 ADAM33 ADAM metallopeptidase domain 33 140492 8756 hCG1789002.2 ADAM7 ADAM metallopeptidase domain 7 122603 101 hCG1816947.1 ADAM8 ADAM metallopeptidase domain 8 183965 8754 hCG1996391 ADAM9 ADAM metallopeptidase domain 9 (meltrin gamma) 129974 27299 hCG15447.3 ADAMDEC1 ADAM-like, -

Integrins: Roles in Cancer Development and As Treatment Targets
British Journal of Cancer (2004) 90, 561 – 565 & 2004 Cancer Research UK All rights reserved 0007 – 0920/04 $25.00 www.bjcancer.com Minireview Integrins: roles in cancer development and as treatment targets 1 ,1,2 H Jin and J Varner* 1John and Rebecca Moores Comprehensive Cancer Center, University of California, San Diego, 9500 Gilman Drive, La Jolla, CA 92093-0912, USA; 2Department of Medicine, University of California, San Diego, 9500 Gilman Drive, La Jolla, CA 92093-0912, USA The integrin family of cell adhesion proteins promotes the attachment and migration of cells on the surrounding extracellular matrix (ECM). Through signals transduced upon integrin ligation by ECM proteins or immunoglobulin superfamily molecules, this family of proteins plays key roles in regulating tumour growth and metastasis as well as tumour angiogenesis. Several integrins play key roles in promoting tumour angiogenesis and tumour metastasis. Antagonists of several integrins (a5b1, avb3 and avb5) are now under evaluation in clinical trials to determine their potential as therapeutics for cancer and other diseases. British Journal of Cancer (2004) 90, 561 – 565. doi:10.1038/sj.bjc.6601576 www.bjcancer.com & 2004 Cancer Research UK Keywords: angiogenesis; metastasis; apoptosis; integrin a5b1; integrin avb3 During the last 10 years, novel insights into the mechanisms sequences (e.g., integrin a4b1 recognises EILDV and REDV in that regulate cell survival as well as cell migration and invasion alternatively spliced CS-1 fibronectin). Inhibitors of integrin have led to the development of novel integrin-based therapeutics function include function-blocking monoclonal antibodies, pep- for the treatment of cancer. Several integrins play important tide antagonists and small molecule peptide mimetics matrix roles in promoting cell proliferation, migration and survival (reviewed in Hynes, 1992; Cheresh, 1993). -

CD Markers Are Routinely Used for the Immunophenotyping of Cells
ptglab.com 1 CD MARKER ANTIBODIES www.ptglab.com Introduction The cluster of differentiation (abbreviated as CD) is a protocol used for the identification and investigation of cell surface molecules. So-called CD markers are routinely used for the immunophenotyping of cells. Despite this use, they are not limited to roles in the immune system and perform a variety of roles in cell differentiation, adhesion, migration, blood clotting, gamete fertilization, amino acid transport and apoptosis, among many others. As such, Proteintech’s mini catalog featuring its antibodies targeting CD markers is applicable to a wide range of research disciplines. PRODUCT FOCUS PECAM1 Platelet endothelial cell adhesion of blood vessels – making up a large portion molecule-1 (PECAM1), also known as cluster of its intracellular junctions. PECAM-1 is also CD Number of differentiation 31 (CD31), is a member of present on the surface of hematopoietic the immunoglobulin gene superfamily of cell cells and immune cells including platelets, CD31 adhesion molecules. It is highly expressed monocytes, neutrophils, natural killer cells, on the surface of the endothelium – the thin megakaryocytes and some types of T-cell. Catalog Number layer of endothelial cells lining the interior 11256-1-AP Type Rabbit Polyclonal Applications ELISA, FC, IF, IHC, IP, WB 16 Publications Immunohistochemical of paraffin-embedded Figure 1: Immunofluorescence staining human hepatocirrhosis using PECAM1, CD31 of PECAM1 (11256-1-AP), Alexa 488 goat antibody (11265-1-AP) at a dilution of 1:50 anti-rabbit (green), and smooth muscle KD/KO Validated (40x objective). alpha-actin (red), courtesy of Nicola Smart. PECAM1: Customer Testimonial Nicola Smart, a cardiovascular researcher “As you can see [the immunostaining] is and a group leader at the University of extremely clean and specific [and] displays Oxford, has said of the PECAM1 antibody strong intercellular junction expression, (11265-1-AP) that it “worked beautifully as expected for a cell adhesion molecule.” on every occasion I’ve tried it.” Proteintech thanks Dr. -

B2 Integrin Signaling Cascade in Neutrophils: More Than a Single Function
REVIEW published: 18 February 2021 doi: 10.3389/fimmu.2020.619925 b2 Integrin Signaling Cascade in Neutrophils: More Than a Single Function † † Panagiota Bouti 1* , Steven D. S. Webbers 1,2* , Susanna C. Fagerholm 3, Ronen Alon 4, Markus Moser 5, Hanke L. Matlung 1 and Taco W. Kuijpers 1,2 1 Sanquin Research and Landsteiner Laboratory, Department of Blood Cell Research, Amsterdam University Medical Center, Edited by: University of Amsterdam, Amsterdam, Netherlands, 2 Department of Pediatric Immunology, Rheumatology and Infectious Vicky L. Morrison, Disease, Amsterdam University Medical Center (AUMC), Emma Children’s Hospital, University of Amsterdam, Amsterdam, University of Glasgow, Netherlands, 3 Research Program of Molecular and Integrative Biosciences, Faculty of Biological and Environmental United Kingdom Sciences, University of Helsinki, Helsinki, Finland, 4 Department of Immunology, The Weizmann Institute of Science, Rehovot, Reviewed by: Israel, 5 Institute of Experimental Hematology, School of Medicine, Technical University of Munich, Munich, Germany Vineet Gupta, Rush University Medical Center, United States Neutrophils are the most prevalent leukocytes in the human body. They have a pivotal role Keehoon Jung, in the innate immune response against invading bacterial and fungal pathogens, while Seoul National University, South Korea recent emerging evidence also demonstrates their role in cancer progression and anti- *Correspondence: Panagiota Bouti tumor responses. The efficient execution of many neutrophil effector responses requires [email protected] the presence of b2 integrins, in particular CD11a/CD18 or CD11b/CD18 heterodimers. Steven D. S. Webbers [email protected] Although extensively studied at the molecular level, the exact signaling cascades †These authors have contributed downstream of b2 integrins still remain to be fully elucidated. -

Cell Adhesion Molecules in Normal Skin and Melanoma
biomolecules Review Cell Adhesion Molecules in Normal Skin and Melanoma Cian D’Arcy and Christina Kiel * Systems Biology Ireland & UCD Charles Institute of Dermatology, School of Medicine, University College Dublin, D04 V1W8 Dublin, Ireland; [email protected] * Correspondence: [email protected]; Tel.: +353-1-716-6344 Abstract: Cell adhesion molecules (CAMs) of the cadherin, integrin, immunoglobulin, and selectin protein families are indispensable for the formation and maintenance of multicellular tissues, espe- cially epithelia. In the epidermis, they are involved in cell–cell contacts and in cellular interactions with the extracellular matrix (ECM), thereby contributing to the structural integrity and barrier for- mation of the skin. Bulk and single cell RNA sequencing data show that >170 CAMs are expressed in the healthy human skin, with high expression levels in melanocytes, keratinocytes, endothelial, and smooth muscle cells. Alterations in expression levels of CAMs are involved in melanoma propagation, interaction with the microenvironment, and metastasis. Recent mechanistic analyses together with protein and gene expression data provide a better picture of the role of CAMs in the context of skin physiology and melanoma. Here, we review progress in the field and discuss molecular mechanisms in light of gene expression profiles, including recent single cell RNA expression information. We highlight key adhesion molecules in melanoma, which can guide the identification of pathways and Citation: D’Arcy, C.; Kiel, C. Cell strategies for novel anti-melanoma therapies. Adhesion Molecules in Normal Skin and Melanoma. Biomolecules 2021, 11, Keywords: cadherins; GTEx consortium; Human Protein Atlas; integrins; melanocytes; single cell 1213. https://doi.org/10.3390/ RNA sequencing; selectins; tumour microenvironment biom11081213 Academic Editor: Sang-Han Lee 1. -

Genetic Testing Medical Policy – Genetics
Genetic Testing Medical Policy – Genetics Please complete all appropriate questions fully. Suggested medical record documentation: • Current History & Physical • Progress Notes • Family Genetic History • Genetic Counseling Evaluation *Failure to include suggested medical record documentation may result in delay or possible denial of request. PATIENT INFORMATION Name: Member ID: Group ID: PROCEDURE INFORMATION Genetic Counseling performed: c Yes c No **Please check the requested analyte(s), identify number of units requested, and provide indication/rationale for testing. 81400 Molecular Pathology Level 1 Units _____ c ACADM (acyl-CoA dehydrogenase, C-4 to C-12 straight chain, MCAD) (e.g., medium chain acyl dehydrogenase deficiency), K304E variant _____ c ACE (angiotensin converting enzyme) (e.g., hereditary blood pressure regulation), insertion/deletion variant _____ c AGTR1 (angiotensin II receptor, type 1) (e.g., essential hypertension), 1166A>C variant _____ c BCKDHA (branched chain keto acid dehydrogenase E1, alpha polypeptide) (e.g., maple syrup urine disease, type 1A), Y438N variant _____ c CCR5 (chemokine C-C motif receptor 5) (e.g., HIV resistance), 32-bp deletion mutation/794 825del32 deletion _____ c CLRN1 (clarin 1) (e.g., Usher syndrome, type 3), N48K variant _____ c DPYD (dihydropyrimidine dehydrogenase) (e.g., 5-fluorouracil/5-FU and capecitabine drug metabolism), IVS14+1G>A variant _____ c F13B (coagulation factor XIII, B polypeptide) (e.g., hereditary hypercoagulability), V34L variant _____ c F2 (coagulation factor 2) (e.g., -

Cardiac Fibrosis: Key Role of Integrins in Cardiac Homeostasis and Remodeling
cells Review Cardiac Fibrosis: Key Role of Integrins in Cardiac Homeostasis and Remodeling Patrick B. Meagher 1,2, Xavier Alexander Lee 1,2 , Joseph Lee 1,2 , Aylin Visram 1,2, Mark K. Friedberg 2,3,4 and Kim A. Connelly 1,2,3,* 1 Keenan Research Centre, Li Ka Shing Knowledge Institute, St. Michael’s Hospital, Toronto, ON M5B 1W8, Canada; [email protected] (P.B.M.); [email protected] (X.A.L.); [email protected] (J.L.); [email protected] (A.V.) 2 Department of Physiology, University of Toronto, Toronto, ON M5S 1A8, Canada; [email protected] 3 Institute of Medical Science, University of Toronto, Toronto, ON M5S 1A8, Canada 4 Labatt Family Heart Center and Department of Paediatrics, Hospital for Sick Children, Toronto, ON M5G 1X8, Canada * Correspondence: [email protected]; Tel.: +141-686-45201 Abstract: Cardiac fibrosis is a common finding that is associated with the progression of heart failure (HF) and impacts all chambers of the heart. Despite intense research, the treatment of HF has primarily focused upon strategies to prevent cardiomyocyte remodeling, and there are no targeted antifibrotic strategies available to reverse cardiac fibrosis. Cardiac fibrosis is defined as an accumulation of extracellular matrix (ECM) proteins which stiffen the myocardium resulting in the deterioration cardiac function. This occurs in response to a wide range of mechanical and biochemical signals. Integrins are transmembrane cell adhesion receptors, that integrate signaling Citation: Meagher, P.B.; Lee, X.A.; between cardiac fibroblasts and cardiomyocytes with the ECM by the communication of mechanical Lee, J.; Visram, A.; Friedberg, M.K.; stress signals. -

Analysis of the Roles of 14-3-3 in the Platelet Glycoprotein Ib-IX–Mediated
Analysis of the Roles of 14-3-3 in the Platelet Glycoprotein Ib-IX–mediated Activation of Integrin aIIbb3 Using a Reconstituted Mammalian Cell Expression Model Minyi Gu,* Xiaodong Xi,* Graham D. Englund,* Michael C. Berndt,‡ and Xiaoping Du* *Department of Pharmacology, University of Illinois College of Medicine, Chicago, Ilinois 60612; and ‡Baker Medical Research Institute, Prahran, VIC 3181, Australia Abstract. We have reconstituted the platelet glycopro- ing to GPIb-IX is important in GPIb-IX signaling. Ex- tein (GP) Ib-IX–mediated activation of the integrin pression of a dominant negative 14-3-3 mutant inhib- aIIbb3 in a recombinant DNA expression model, and ited cell spreading on vWF, suggesting an important show that 14-3-3 is important in GPIb-IX signaling. role for 14-3-3. Deleting both the 14-3-3 and filamin- CHO cells expressing aIIbb3 adhere poorly to vWF. binding sites of GPIba induced an endogenous inte- Cells expressing GPIb-IX adhere to vWF in the pres- grin-dependent cell spreading on vWF without requir- ence of botrocetin but spread poorly. Cells coexpress- ing aIIbb3, but inhibited vWF-induced fibrinogen ing integrin aIIbb3 and GPIb-IX adhere and spread on binding to aIIbb3. Thus, while different activation mech- vWF, which is inhibited by RGDS peptides and anti- anisms may be responsible for vWF interaction with bodies against aIIbb3. vWF binding to GPIb-IX also ac- different integrins, GPIb-IX–mediated activation of tivates soluble fibrinogen binding to aIIbb3 indicating aIIbb3 requires 14-3-3 interaction with GPIba. that GPIb-IX mediates a cellular signal leading to aIIbb3 activation. -
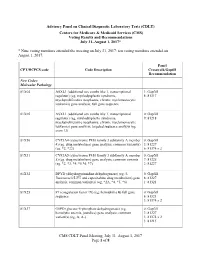
Advisory Panel on Clinical Diagnostic Laboratory Tests
Advisory Panel on Clinical Diagnostic Laboratory Tests (CDLT) Centers for Medicare & Medicaid Services (CMS) Voting Results and Recommendations July 31–August 1, 2017* * Nine voting members attended the meeting on July 31, 2017; ten voting members attended on August 1, 2017. Panel CPT/HCPCS code Code Description Crosswalk/Gapfill Recommendation New Codes: Molecular Pathology 81X04 ASXL1 (additional sex combs like 1, transcriptional 1: Gapfill regulator) (eg, myelodysplastic syndrome, 8: 81317 myeloproliferative neoplasms, chronic myelomonocytic leukemia) gene analysis; full gene sequence 81X05 ASXL1 (additional sex combs like 1, transcriptional 0: Gapfill regulator) (eg, myelodysplastic syndrome, 9: 81218 myeloproliferative neoplasms, chronic myelomonocytic leukemia) gene analysis; targeted sequence analysis (eg, exon 12) 81X30 CYP3A4 (cytochrome P450 family 3 subfamily A member 0: Gapfill 4) (eg, drug metabolism) gene analysis, common variant(s) 3: 81227 (eg, *2, *22) 6: 81374 x 2 81X31 CYP3A5 (cytochrome P450 family 3 subfamily A member 0: Gapfill 5) (eg, drug metabolism) gene analysis, common variants 7: 81225 (eg, *2, *3, *4, *5 *6, *7) 2: 81227 81X32 DPYD (dihydropyrimidine dehydrogenase) (eg, 5- 0: Gapfill fluorouracil/5-FU and capecitabine drug metabolism) gene 8: 81227 analysis, common variant(s) (eg, *2A, *4, *5, *6) 1: 81321 81X25 F9 (coagulation factor IX) (eg, hemophilia B) full gene 0: Gapfill sequence 6: 81321 3: 81374 x 2 81X37 G6PD (glucose-6-phosphate dehydrogenase) (eg, 0: Gapfill hemolytic anemia, jaundice) gene analysis; -

Cell Adhesion Molecules Are Mediated by Photobiomodulation at 660 Nm in Diabetic Wounded Fibroblast Cells
cells Article Cell Adhesion Molecules Are Mediated by Photobiomodulation at 660 nm in Diabetic Wounded Fibroblast Cells Nicolette N. Houreld * ID , Sandra M. Ayuk and Heidi Abrahamse ID Laser Research Centre, Faculty of Health Sciences, University of Johannesburg, P.O. Box 17011, Doornfontein, Johannesburg 2028, South Africa; [email protected] (S.M.A.); [email protected] (H.A.) * Correspondence: [email protected]; Tel.: +27-11-559-6833 Received: 9 March 2018; Accepted: 12 April 2018; Published: 16 April 2018 Abstract: Diabetes affects extracellular matrix (ECM) metabolism, contributing to delayed wound healing and lower limb amputation. Application of light (photobiomodulation, PBM) has been shown to improve wound healing. This study aimed to evaluate the influence of PBM on cell adhesion molecules (CAMs) in diabetic wound healing. Isolated human skin fibroblasts were grouped into a diabetic wounded model. A diode laser at 660 nm with a fluence of 5 J/cm2 was used for irradiation and cells were analysed 48 h post-irradiation. Controls consisted of sham-irradiated (0 J/cm2) cells. Real-time reverse transcription (RT) quantitative polymerase chain reaction (qPCR) was used to determine the expression of CAM-related genes. Ten genes were up-regulated in diabetic wounded cells, while 25 genes were down-regulated. Genes were related to transmembrane molecules, cell–cell adhesion, and cell–matrix adhesion, and also included genes related to other CAM molecules. PBM at 660 nm modulated gene expression of various CAMs contributing to the increased healing seen in clinical practice. There is a need for new therapies to improve diabetic wound healing. -

101 CMR 320.00 Clinical Laboratory Services
The Commonwealth of Massachusetts Executive Office of Health and Human Services One Ashburton Place, Room 1109 Boston, Massachusetts 02108 CHARLES D. BAKER Tel: (617) 573-1600 Governor Fax: (617) 573-1891 www.mass.gov/eohhs KARYN E. POLITO Lieutenant Governor MARYLOU SUDDERS Secretary Administrative Bulletin 18-23 101 CMR 320.00 Clinical Laboratory Services Effective January 1, 2018 Procedure Code Update Under the authority of regulation 101 CMR 320.01(3), the Executive Office of Health and Human Services is adding new procedure codes and deleting outdated codes. Per the regulation, the rates for code additions are priced at 74.67% of the prevailing Medicare rate, if available. If Medicare rates are unavailable, services are priced at individual consideration. The rate for new code replacements were set at the rate previously set for the replaced codes. The changes, effective January 1, 2018, are as follows. Code Change Rate Code Description (if applicable) Human Platelet Antigen 1 genotyping (HPA-1), ITGB3 (integrin, beta 3 [platelet glycoprotein IIIa], antigen CD61 [GPIIIa]) (e.g., neonatal alloimmune 81105 Addition $112.67 thrombocytopenia [NAIT], post-transfusion purpura), gene analysis, common variant, HPA-1a/b (L33P) Human Platelet Antigen 2 genotyping (HPA-2), GP1BA (glycoprotein Ib [platelet], alpha polypeptide [GPIba]) (e.g., neonatal alloimmune 81106 Addition $112.67 thrombocytopenia [NAIT], post-transfusion purpura), gene analysis, common variant, HPA-2a/b (T145M) Human Platelet Antigen 3 genotyping (HPA-3), ITGA2B (integrin -

Genetics (Alphabetic), Associated Disease, Laboratory and Clinical Picture, Together with Special Features of Inherited Platelet Defects (Status: März 2020)
Genetics (alphabetic), associated disease, laboratory and clinical picture, together with special features of inherited platelet defects (Status: März 2020) Compiled by: Werner Streif (Pädiatrie I, Medical University of Innsbruck, Austria), Jennifer Gebetsberger (Pädiatrie I, Medical University of Innsbruck, Austria), Reviewed by: Georgi Manukjan (Experimentelle Biomedizin, Universitätsklinikum Würzburg, Germany), Harald Schulze (Experimentelle Biomedizin, Universitätsklinikum Würzburg, Germany) Defect Abbreviation, Synonym Disease Inheritance Gene OMIM # Phenotype OMIM# Gene location # Size BT S P Special features (Background, Laboratory, Clinic) Sitosterolemia, also known as phytosterolemia, is an autosomal recessive metabolic condition characterized by unrestricted intestinal absorption of both ATP-BINDING CASSETTE, SUBFAMILY G, MEMBER ABCG5 Sitosterolemia with macrothrombocytopenia AR 605459 618666 2p21 r l / S / cholesterol and plant-derived cholesterol-like molecules, such as sitosterol. 5; STEROLIN 1 Patients with this disorder have very high levels of plant sterols in the plasma and develop tendon and tuberous xanthomas, accelerated atherosclerosis, and premature coronary artery disease. Sitosterolemia, also known as phytosterolemia, is an autosomal recessive metabolic condition characterized by unrestricted intestinal absorption of both ATP-BINDING CASSETTE, SUBFAMILY G, MEMBER ABCG8 Sitosterolemia with macrothrombocytopenia AR 605460 210250 2p21 r l / S / cholesterol and plant-derived cholesterol-like molecules, such as sitosterol.