Fish Exploitation at the Sea of Galilee (Israel) by Early Fisher
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Acanthobrama Telavivensis), in a Mediterranean-Type Stream
Environ Biol Fish DOI 10.1007/s10641-006-9066-8 ORIGINAL PAPER Reproductive strategy of a small endemic cyprinid, the Yarqon bleak (Acanthobrama telavivensis), in a mediterranean-type stream Eldad Elron Æ Avital Gasith Æ Menachem Goren Received: 22 November 2005 / Accepted: 4 April 2006 Ó Springer Science+Business Media B.V. 2006 Abstract A mediterranean-type climate exists in mediterranean-climate hydrologic regime. It five widely separated regions; the Mediterranean breeds in late winter and early spring, a window basin, parts of western North America, parts of of opportunity between flash floods and habitat western and southern Australia, southwestern desiccation. Being a multiple spawner allows the South Africa and parts of central Chile. Streams fish to compensate for the potential loss of part of in these regions feature seasonal disturbances of its reproductive output due to scouring flows of contrasting hydrology with high predictability of late floods. The ability of the Yarqon bleak to the timing of flooding and drying but low con- spawn on different substrate-types enables it to stancy. We would expect fish living in these take advantage of different stream conditions that streams to avoid scouring flow and breed after pertain in different years. The fish attains pre- cessation of the flood period. The aim of the adult size (ca. 33–42 mm) within the first year, present study was to examine the adaptation of prior to drying out of most stream reaches, and the Yarqon bleak, Acanthobrama telavivensis,an matures by the beginning of the second year endemic cyprinid in the coastal streams of Israel, (males >41; females >42 mm). -

KEY WORDS: Maritime Cultural Heritage; Israel's Seas
MANAGEMENT OF THE UNDERWATER AND COASTAL ARCHAEOLOGICAL HERITAGE IN ISRAEL’S SEAS (I) GESTIÓN DEL PATRIMONIO ARQUEOLÓGICO SUBACUÁTICO Y COSTERO EN LOS MARES DE ISRAEL (I) EHUD GALILI1 - SARAH ARENSON2 [email protected] [email protected] ABSTRACT The Maritime cultural heritage of Israel reflects important chapters in the history of humanity, including the Neolithic revolution and the beginning of agriculture, the emergence of the first empires and the foundation of the three monotheistic religions. Erosion due to sea level rise and human activity is destroying important coastal and underwater archaeological sites. Low levels in the Sea of Galilee and the Dead Sea exposed many archaeological remains, which are threatened by erosion, treasure hunting and coastal development. Without intensive rescue and protection activities, the valuable cultural maritime heritage will vanish within a few decades. Legal and operational tools are currently 151 applied in order to assure integrated coastal zone management, to control coastal development and to protect the marine cultural resources. Among these are the Antiquities Law, the Planning and Building Law, the Nature and Parks Law and the Law for the Protection of the Coastal Environment. Coastal and underwater sites are mapped and monitored, protected coastal and underwater sites are declared and rescue surveys are carried out in the endangered sites. A national risk assessment document of the coastal and underwater heritage and detailed preservation and protection plans have been prepared. Pilot projects for protecting and preserving the sea walls of Ashkelon, Apollonia and Akko were completed and several damaged harbors in the Sea of Galilee were reconstructed. -

Length-Weight Relationship of Ten Species of Leuciscinae Sub-Family (Cyprinidae) from Iranian Inland Waters
Int Aquat Res (2020) 12:133–136 https://doi.org/10.22034/IAR(20).2020.1891648.1004 SHORT COMMUNICATION Length-weight relationship of ten species of Leuciscinae sub-family (Cyprinidae) from Iranian inland waters Soheil Eagderi . Atta Mouludi-Saleh . Erdogan Çiçek Received: 23 January 2020 / Accepted: 16 April 2020 / Published online: 16 May 2020 © The Author(s) 2020 Abstract In this study, the length-weight relationships (LWRs) were estimated for 697 specimens belonging to ten species of Leuciscinae subfamily, viz. Squalius berak, S. namak, S. turcicus, Acanthobrama marmid, A. microlepis, Chondrostoma esmaeilii, Leuciscus latus, Alburnus doriae, Leucaspius delineates and Blicca bjoerkna from Iranian inland waters, including the Persian Gulf, Caspian Sea, Namak Lake, Dasht-e Kavir, Hari River and Isfahan basins. Total length and total weight of the examined specimens ranged 3.8-27.8 cm and 0.59-247 g, respectively. Fishes were collected using electrofishing device between July 2010 and August 2017. This study represents the first reports of LWRs data for A. doriae, C. esmaeilii, L. latus and L. delineates. The length-weight parameter b found to be 2.75 (A. microlepis) - 3.44 (B. bjoerkna) with regression coefficients (r2) ranging 0.87 to 0.99 for the studied fishes. The student’s t-test showed all studied species have a positive allometric growth pattern except A. doriae (isometric) and A. microlepis (negative allometric). Keywords LWRs . Leuciscinae . Chondrostoma esmaeilii . Leuciscus latus Introduction Study of fish species and their populations are important to understand their biological aspects in order to optimal management of their resources (Jalili et al. -

Age, Growth and Mortality of Acanthobrama Microlepis (De Filippi, 1863) from Lake Çıldır, Turkey
Age, growth and mortality of Acanthobrama microlepis (De Filippi, 1863) from Lake Çıldır, Turkey Nurcihan ÇAKIR, Erdoğan ÇİÇEK*, Burak SEÇER, Sevil BİRECİKLİGİL Department of Biology, Faculty of Art and Sciences, Nevşehir Hacı Bektaş Veli University, Nevşehir, Turkey. *Corresponding author: [email protected] Abstract: This study was carried out in August-September 2014 and May 2015 in Lake Çıldır. A total of 229 specimens of Acanthobrama microlepis were used to determine some population parameters. The age of the specimens ranged from I to IV age groups and dominant age group was II. Total length varied from 7.6 to 24.2 cm with mean of 16.23±4.22 cm and total weight ranged from 3.68 to 123.46 g with mean of 42.70±33.24 g. The length- 3.1199 weight relationship were estimated as W = 0.0058L . The von Bertalanffy growth parameters, growth performance index and Fulton’s condition factor were calculates as L∞ = -1 38.37 cm, k = 0.193 year , to = -0.73 year, Φ = 2.45 and K = 0.75, respectively. Total mortality, natural mortality, fishing mortality and exploitation rates also estimated as Z = 0.50, M = 0.28, F = 0.22 and E = 0.44, respectively. In the light of these values it could not indicate any overfishing on population based on the exploitation rate. Keywords: Acanthobrama microlepis, Blackbrow bleak, Caucasian bream, Lake Çıldır, Population parameters, Mortality rates. Introduction Coad, 2015). Even though the species has not economic The genus Acanthalburnus belongs to Leuciscine value, is used for human consumption by locally, Cyprinids and distributed in Southwest Asia. -

Migration of Eretz Yisrael Arabs Between December 1, 1947 and June 1, 1948
[Intelligence Service (Arab Section)] June 30, 1948 Migration of Eretz Yisrael Arabs between December 1, 1947 and June 1, 1948 Contents 1. General introduction. 2. Basic figures on Arab migration 3. National phases of evacuation and migration 4. Causes of Arab migration 5. Arab migration trajectories and absorption issues Annexes 1. Regional reviews analyzing migration issues in each area [Missing from document] 2. Charts of villages evacuated by area, noting the causes for migration and migration trajectories for every village General introduction The purpose of this overview is to attempt to evaluate the intensity of the migration and its various development phases, elucidate the different factors that impacted population movement directly and assess the main migration trajectories. Of course, given the nature of statistical figures in Eretz Yisrael in general, which are, in themselves, deficient, it would be difficult to determine with certainty absolute numbers regarding the migration movement, but it appears that the figures provided herein, even if not certain, are close to the truth. Hence, a margin of error of ten to fifteen percent needs to be taken into account. The figures on the population in the area that lies outside the State of Israel are less accurate, and the margin of error is greater. This review summarizes the situation up until June 1st, 1948 (only in one case – the evacuation of Jenin, does it include a later occurrence). Basic figures on Arab population movement in Eretz Yisrael a. At the time of the UN declaration [resolution] regarding the division of Eretz Yisrael, the following figures applied within the borders of the Hebrew state: 1. -
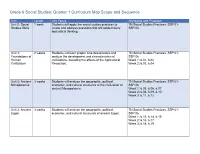
Grade 6 Social Studies: Quarter 1 Curriculum Map Scope and Sequence
Grade 6 Social Studies: Quarter 1 Curriculum Map Scope and Sequence Unit Length Unit Focus Standards and Practices Unit 0: Social 1 week Students will apply the social studies practices to TN Social Studies Practices: SSP.01- Studies Skills create and address questions that will guide inquiry SSP.06 and critical thinking. Unit 1: 2 weeks Students will learn proper time designations and TN Social Studies Practices: SSP.01- Foundations of analyze the development and characteristics of SSP.06 Human civilizations, including the effects of the Agricultural Week 1: 6.01, 6.02 Civilization Revolution. Week 2: 6.03, 6.04 Unit 2: Ancient 3 weeks Students will analyze the geographic, political, TN Social Studies Practices: SSP.01- Mesopotamia economic, and cultural structures of the civilization of SSP.06 ancient Mesopotamia. Week 1: 6.05, 6.06, 6.07 Week 2: 6.08, 6.09, 6.10 Week 3: 6.11, 6.12 Unit 3: Ancient 3 weeks Students will analyze the geographic, political, TN Social Studies Practices: SSP.01- Egypt economic, and cultural structures of ancient Egypt. SSP.06 Week 1: 6.13, 6.14, 6.15 Week 2: 6.16, 6.17 Week 3: 6.18, 6.19 Grade 6 Social Studies: Quarter 1 Map Instructional Framework Course Description: World History and Geography: Early Civilizations Through the Fall of the Western Roman Empire Sixth grade students will study the beginnings of early civilizations through the fall of the Western Roman Empire. Students will analyze the cultural, economic, geographical, historical, and political foundations for early civilizations, including Mesopotamia, Egypt, Israel, India, China, Greece, and Rome. -

Women's Position and Attitudes Towards Female Genital Mutilation in Egypt
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Springer - Publisher Connector Van Rossem et al. BMC Public Health (2015) 15:874 DOI 10.1186/s12889-015-2203-6 RESEARCH ARTICLE Open Access Women's position and attitudes towards female genital mutilation in Egypt: A secondary analysis of the Egypt demographic and health surveys, 1995-2014 Ronan Van Rossem1*, Dominique Meekers2 and Anastasia J. Gage2 Abstract Background: Female genital mutilation (FGM) is still widespread in Egyptian society. It is strongly entrenched in local tradition and culture and has a strong link to the position of women. To eradicate the practice a major attitudinal change is a required for which an improvement in the social position of women is a prerequisite. This study examines the relationship between Egyptian women’s social positions and their attitudes towards FGM, and investigates whether the spread of anti-FGM attitudes is related to the observed improvements in the position of women over time. Methods: Changes in attitudes towards FGM are tracked using data from the Egypt Demographic and Health Surveys from 1995 to 2014. Multilevel logistic regressions are used to estimate 1) the effects of indicators of a woman’ssocial position on her attitude towards FGM, and 2) whether these effects change over time. Results: Literate, better educated and employed women are more likely to oppose FGM. Initially growing opposition to FGM was related to the expansion of women’s education, but lately opposition to FGM also seems to have spread to other segments of Egyptian society. -

Application of the RAPD Technique in Tilapia Fish: Species and Subspecies Identification
Heredity 73(1994) 117—123 Received 25 November 1993 Genetical Society of Great Britain Application of the RAPD technique in tilapia fish: species and subspecies identification F. BARDAKCI* & D. 0. F. SKIBINSKI Molecular Biology Research Group, School of Biological Sciences, University of Wales, Singleton Park, Swansea SA2 8PP, U.K. RandomAmplified Polymorphic DNA (RAPD) analysis was applied to three species of the tilapia genus Oreochromis and four subspecies of 0. niloticus. Thirteen random lO-mer primers were used to assay polymorphisms within and between populations. Different RAPD fragment patterns were observed for different species, although not always for different subspecies. Evidence is presented that RAPD markers might be useful for systematic investigation at the level of species and subspecies. Keywords:DNA,Oreochromis, polymorphism, RAPD, systematics, tilapia. fully to identify the subspecies of 0. niloticus (Capili, Introduction 1990; Seyoum & Kornfield, 1992) but little effort has Tilapiaare cultured extensively throughout the world, yet been devoted to the analysis of nuclear DNA for especially in Africa and the Far East. Most of the this purpose. important tilapiine species used in aquaculture are The development of random amplified polymorphic members of the Oreochromis genus, as many members DNA (RAPD) markers, generated by the polymerase of this taxon grow well under diverse culture condi- chain reaction (PCR), allows the examination of tions (Fryer & lIes, 1972; Pullin & Capili, 1989). genomic variation without prior knowledge of DNA Despite its commercial importance, resources have sequences (Williams et a!., 1990, 1993; Welsh & only recently been devoted to the development of McClelland, 1990; Hadrys, 1992). The number and improved strains of tilapia. -

Phylogenetic Relationships of Freshwater Fishes of the Genus Capoeta (Actinopterygii, Cyprinidae) in Iran
Received: 3 May 2016 | Revised: 8 August 2016 | Accepted: 9 August 2016 DOI: 10.1002/ece3.2411 ORIGINAL RESEARCH Phylogenetic relationships of freshwater fishes of the genus Capoeta (Actinopterygii, Cyprinidae) in Iran Hamid Reza Ghanavi | Elena G. Gonzalez | Ignacio Doadrio Museo Nacional de Ciencias Naturales, Biodiversity and Evolutionary Abstract Biology Department, CSIC, Madrid, Spain The Middle East contains a great diversity of Capoeta species, but their taxonomy re- Correspondence mains poorly described. We used mitochondrial history to examine diversity of the Hamid Reza Ghanavi, Department of algae- scraping cyprinid Capoeta in Iran, applying the species- delimiting approaches Biology, Lund University, Lund, Sweden. Email: [email protected] General Mixed Yule- Coalescent (GMYC) and Poisson Tree Process (PTP) as well as haplotype network analyses. Using the BEAST program, we also examined temporal divergence patterns of Capoeta. The monophyly of the genus and the existence of three previously described main clades (Mesopotamian, Anatolian- Iranian, and Aralo- Caspian) were confirmed. However, the phylogeny proposed novel taxonomic findings within Capoeta. Results of GMYC, bPTP, and phylogenetic analyses were similar and suggested that species diversity in Iran is currently underestimated. At least four can- didate species, Capoeta sp4, Capoeta sp5, Capoeta sp6, and Capoeta sp7, are awaiting description. Capoeta capoeta comprises a species complex with distinct genetic line- ages. The divergence times of the three main Capoeta clades are estimated to have occurred around 15.6–12.4 Mya, consistent with a Mio- Pleistocene origin of the di- versity of Capoeta in Iran. The changes in Caspian Sea levels associated with climate fluctuations and geomorphological events such as the uplift of the Zagros and Alborz Mountains may account for the complex speciation patterns in Capoeta in Iran. -

The KHALUB-Tree in Mesopotamia: Myth Or Reality?
University of Pennsylvania ScholarlyCommons University of Pennsylvania Museum of University of Pennsylvania Museum of Archaeology and Anthropology Papers Archaeology and Anthropology 2009 The KHALUB-tree in Mesopotamia: Myth or Reality? Naomi F. Miller University of Pennsylvania, [email protected] Alhena Gadotti Follow this and additional works at: https://repository.upenn.edu/penn_museum_papers Part of the Archaeological Anthropology Commons Recommended Citation (OVERRIDE) Miller, N.F. & Gadotti, A. (2009). The KHALUB-tree in Mesopotamia: Myth or Reality? In A.S. Fairbairn & E. Weiss (Eds.). From Foragers to Farmers: Gordon C. Hillman Festschrift (pp. 239-243). Oxford: Oxbow Books. This paper is posted at ScholarlyCommons. https://repository.upenn.edu/penn_museum_papers/21 For more information, please contact [email protected]. The KHALUB-tree in Mesopotamia: Myth or Reality? Disciplines Archaeological Anthropology This book chapter is available at ScholarlyCommons: https://repository.upenn.edu/penn_museum_papers/21 This pdf of your paper in Foragers and Farmers belongs to the publishers Oxbow Books and it is their copyright. As author you are licenced to make up to 50 offprints from it, but beyond that you may not publish it on the World Wide Web until three years from publication (June 20 12), unless the site is a limited access intranet (pass word protected). If you have queries about this please contact the editorial department at Oxbow Books ([email protected]). An Offprint from FRoM FoRAGERS TO FARMERS GoRDON C. HILLMAN FESTSCHRIFT Edited by AndrewS. Fairbairn and Ehud Weiss OXBOW BOOKS Oxford and Oakville Contents Introduction: In honour of Professor Gordon C. Hillman .... ........... ....... ............. .. ... .. ....................... ... .......... .. ... vn Publications of Gordon C. -

New Data on the Distribution and Conservation Status of the Two Endemic Scrapers in the Turkish Mediterranean Sea Drainages (Teleostei: Cyprinidae)
International Journal of Zoology and Animal Biology ISSN: 2639-216X New Data on the Distribution and Conservation Status of the Two Endemic Scrapers in the Turkish Mediterranean Sea Drainages (Teleostei: Cyprinidae) Kaya C1*, Kucuk F2 and Turan D1 Research Article 1 Recep Tayyip Erdogan University, Turkey Volume 2 Issue 6 2Isparta University of Applied Sciences, Turkey Received Date: October 31, 2019 Published Date: November 13, 2019 *Corresponding author: Cuneyt Kaya, Faculty of Fisheries and Aquatic Sciences, DOI: 10.23880/izab-16000185 Recep Tayyip Erdogan University, 53100 Rize, Turkey, Tel: +904642233385; Email: [email protected] Abstract In the scope of this study, exact distribution of the two endemic Capoeta species in the Turkish Mediterranean Sea drainages was presented. Fishes were caught with pulsed DC electro-fishing equipment from 28 sampling sites throughout Turkish Mediterranean Sea drainages between Göksu River and stream Boğa. The findings of the study demonstrate that Capoeta antalyensis inhabits in Köprüçay and Aksu rivers, and streams Boğa and Gündoğdu, all around Antalya. Capoeta caelestis widely distributed in coastal stream and rivers between Stream Dim (Alanya) in the west and Göksu River (Silifke) in the east. Metric and meristic characters were collected from the fish samples which obtained in the field for Capoeta caelestis and Capoeta antalyensis, and museum material for Capoeta damascina. In this way, morphologic features of the species revealed and Capoeta caelestis compared with Capoeta damascina to remove the hesitations about the validity of the species. The conservation status of Capoeta antalyensis was recommended to uplist from Vulnerable to Endangered. Keywords: Freshwater Fish Species; Anatolia; Pisces; Capoeta antalyensis; C. -

© in This Web Service Cambridge University
Cambridge University Press 978-1-107-01829-7 - Human Adaptation in the Asian Palaeolithic: Hominin Dispersal and Behaviour during the Late Quaternary Ryan J. Rabett Index More information Index Abdur, 88 Arborophilia sp., 219 Abri Pataud, 76 Arctictis binturong, 218, 229, 230, 231, 263 Accipiter trivirgatus,cf.,219 Arctogalidia trivirgata, 229 Acclimatization, 2, 7, 268, 271 Arctonyx collaris, 241 Acculturation, 70, 279, 288 Arcy-sur-Cure, 75 Acheulean, 26, 27, 28, 29, 45, 47, 48, 51, 52, 58, 88 Arius sp., 219 Acheulo-Yabrudian, 48 Asian leaf turtle. See Cyclemys dentata Adaptation Asian soft-shell turtle. See Amyda cartilaginea high frequency processes, 286 Asian wild dog. See Cuon alipinus hominin adaptive trajectories, 7, 267, 268 Assamese macaque. See Macaca assamensis low frequency processes, 286–287 Athapaskan, 278 tropical foragers (Southeast Asia), 283 Atlantic thermohaline circulation (THC), 23–24 Variability selection hypothesis, 285–286 Attirampakkam, 106 Additive strategies Aurignacian, 69, 71, 72, 73, 76, 78, 102, 103, 268, 272 economic, 274, 280. See Strategy-switching Developed-, 280 (economic) Proto-, 70, 78 technological, 165, 206, 283, 289 Australo-Melanesian population, 109, 116 Agassi, Lake, 285 Australopithecines (robust), 286 Ahmarian, 80 Azilian, 74 Ailuropoda melanoleuca fovealis, 35 Airstrip Mound site, 136 Bacsonian, 188, 192, 194 Altai Mountains, 50, 51, 94, 103 Balobok rock-shelter, 159 Altamira, 73 Ban Don Mun, 54 Amyda cartilaginea, 218, 230 Ban Lum Khao, 164, 165 Amyda sp., 37 Ban Mae Tha, 54 Anderson, D.D., 111, 201 Ban Rai, 203 Anorrhinus galeritus, 219 Banteng. See Bos cf. javanicus Anthracoceros coronatus, 219 Banyan Valley Cave, 201 Anthracoceros malayanus, 219 Barranco Leon,´ 29 Anthropocene, 8, 9, 274, 286, 289 BAT 1, 173, 174 Aq Kupruk, 104, 105 BAT 2, 173 Arboreal-adapted taxa, 96, 110, 111, 113, 122, 151, 152, Bat hawk.