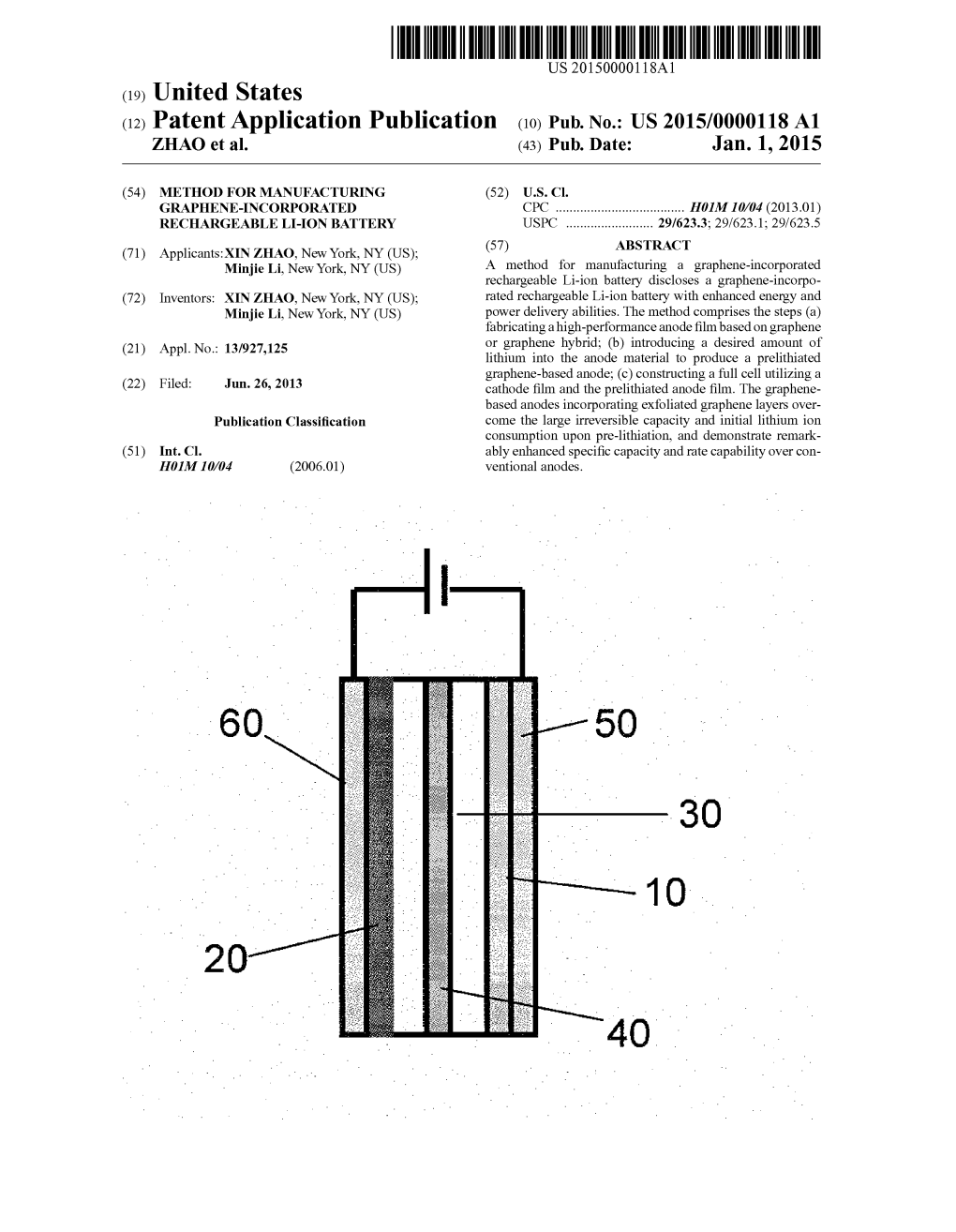
(12) Patent Application Publication (10) Pub. No.: US 2015/0000118A1 ZHAO Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

United States Patent (19) (11) Patent Number: 4,859,572 Farid Et Al
United States Patent (19) (11) Patent Number: 4,859,572 Farid et al. (45) Date of Patent: Aug. 22, 1989 54 DYE SENSTIZED PHOTOGRAPHIC IMAGING SYSTEM FOREIGN PATENT DOCUMENTS 0223587A 5/1987 European Pat. Off, . (75) Inventors: Samir Y. Farid; Roger E. Moody, 2083832A 3/1982 United Kingdom . both of Rochester, N.Y. OTHER PUBLICATIONS (73) Assignee: Eastman Kodak Company, Volman et al., Advances in Photochemistry, vol. 13, in the Rochester, N.Y. chapter titled "Dye Sensitized Photopolymerization' 21 Appl. No.: 189,002 by D. F. Eaton, pp. 427 to 488, John Wiley & Sons (1986). 22) Filed: May 2, 1988 Farid et al., U.S. Ser. Nos. 933,712, 933,658,933,660, 51 Int. Cl." ................................................ G03C 1/68 and 933,657, all filed Nov. 21, 1986, and commonly (52) U.S. Cl. ..... ... 430/281; 430/914; assigned (Now issued as Farid et al U.S. Pat. Nos. 430/915; 430/916; 522/25; 522/26; 522/27; 4,743,528; 4,743,529; 4,743,530; and 4,743,531). 522/28; 522/31 Primary Examiner-Paul R. Michl (58) Field of Search ............... 430/915, 281, 916,914; Assistant Examiner-C. D. R. Dee 522/25, 28, 63, 27, 26, 31 Attorney, Agent, or Firm-Carl O. Thomas 56) References Cited (57) ABSTRACT U.S. PATENT DOCUMENTS A photographic imaging system is disclosed comprised 4,050,934 9/1977 Turner .................................. 96/1 R of a hardenable organic component containing ethyl 4,289,842 9/1981 Tan et al. ... ... 430/270 enic unsaturation sites and an initiator system for ethyl 4,307,182 12/1981 Dalzell et al. -

Emission Load of Car Service Interiors
Vol. 62, 2016 (3): 122–128 Res. Agr. Eng. doi: 10.17221/5/2015-RAE Emission load of car service interiors I. Vitázek1, D. Michalíková1, B. Vitázková2, J. Klúčik1 1Department of Transport and Handling, Faculty of Engineering, Slovak University of Agriculture in Nitra, Nitra, Slovak Republic 2Department of Machines and Production Systems, Faculty of Engineering, Slovak University of Agriculture in Nitra, Nitra, Slovak Republic Abstract Vitázek I., Michalíková D., Vitázková B., Klúčik J. (2016): Emission load of car service interiors. Res. Agr. Eng., 62: 122–128. Car service centres are specific in terms of production of pollutants. The aim of the paper is to assess the quality of indoor environment of car service interiors with respect to the safe range of oxocarbon emission limits, concentration of gaseous and solid aerosols of selected chemical pollutants and occupational noise exposure. Measurements of concen- tration and exposure time indicated that the permitted limits were kept. CO concentration reached values in the range from 0 to 10 ppm, CO2 concentration was observed in the range from 493 to 967 ppm. Concentration of solid aerosol of polyester bitumen reached the maximum value of 0.37 mg/m3, while for gaseous aerosol (e.g. toluene) it equalled 8.114 mg/m3. Measurements of chemical factors were carried out and evaluated by companies with appropriate accredi- tation. Occupational Exposure Limits (OELs) were higher in case of all selected substances. OEL was not demonstrably exceeded at any chemical factor. Noise emissions approached the limit values; therefore, hearing protection is required. Keywords: indoor environment; gaseous emissions; chemical factor; emission limits; noise When undertaking the occupation, time spent in climate of workplaces, residential premises and the workplace may add up to more than a half of the other spaces. -

Some Unusual, Astronomically Significant Organic Molecules
'lL-o Thesis titled: Some Unusual, Astronomically Significant Organic Molecules submitted for the Degree of Doctor of Philosophy (Ph,D.) by Salvatore Peppe B.Sc. (Hons.) of the Department of Ghemistty THE UNIVERSITY OF ADELAIDE AUSTRALIA CRUC E June2002 Preface Gontents Contents Abstract IV Statement of Originality V Acknowledgments vi List of Figures..... ix 1 I. Introduction 1 A. Space: An Imperfect Vacuum 1 B. Stellff Evolution, Mass Outflow and Synthesis of Molecules 5 C. Astronomical Detection of Molecules......... l D. Gas Phase Chemistry.. 9 E. Generation and Detection of Heterocumulenes in the Laboratory 13 L.2 Gas Phase Generation and Characterisation of Ions.....................................16 I. Gas Phase Generation of Ions. I6 A. Positive Ions .. I6 B. Even Electron Negative Ions 17 C. Radical Anions 2t tr. Mass Spectrometry 24 A. The VG ZAB 2}lF Mass Spectrometer 24 B. Mass-Analysed Ion Kinetic Energy Spectrometry......... 25 III. Characterisation of Ions.......... 26 A. CollisionalActivation 26 B. Charge Reversal.... 28 C. Neutralisation - Reionisation . 29 D. Neutral Reactivity. JJ rv. Fragmentation Behaviour ....... 35 A. NegativeIons.......... 35 Preface il B. Charge Inverted Ions 3l 1.3 Theoretical Methods for the Determination of Molecular Geometries and Energetics..... ....o........................................ .....39 L Molecular Orbital Theory........ 39 A. The Schrödinger Equation.... 39 B. Hartree-Fock Theory ..44 C. Electron Correlation ..46 D. Basis sets............ .51 IL Transition State Theory of Unimolecular Reactions ......... ................... 54 2. Covalently Bound Complexes of CO and COz ....... .........................58 L Introduction 58 tr. Results and Discussion........... 59 Part A: Covalently bound COz dimers (OzC-COr)? ............ 59 A. Generation of CzO¿ Anions 6I B. NeutralCzO+........ -

Assessment of Carbon Monoxide (Co) Level in Enugu Metropolis Monitoring Industrial and Residential Area
ASSESSMENT OF CARBON MONOXIDE (CO) LEVEL IN ENUGU METROPOLIS MONITORING INDUSTRIAL AND RESIDENTIAL AREA BY ADIKE JOSEPH .N. CHE/2007/123 PROJECT REPORT SUBMITTED TO THE DEPARTMENT OF CHEMICAL ENGINEERING FACULTY OF ENGINEERING CARITAS UNIVERSITY, AMORJI-NIKE ENUGU STATE AUGUST, 2012 TITLE PAGE ASSESSMENT OF CARBON MONOXIDE (CO) LEVEL IN ENUGU METROPOLIS MONITORING INDUSTRIAL AND RESIDENTIAL AREA BY ADIKE JOSEPH .N. CHE/2007/123 PROJECT REPORT SUBMITTED TO THE DEPARTMENT OF CHEMICAL ENGINEERING, FACULTY OF ENGINEERING IN PARTIAL FULFILLMENT OF THE REQUIREMENT FOR THE AWARD OF BACHELOR OF ENGINEERING, DEGREE IN CHEMICAL CARITAS UNIVERSITY AMORJI-NIKE ENUGU STATE AUGUST, 2012 CERTIFICATION This is to certify that this work was down under the supervision of Supervisor‟s name Engr Ken Ezeh Signature------------------ ------------------------------ date ------------------------------ Head of Department ------------------------------------------------------------------------ Signature ------------------------------------------------ Date ------------------------------ External supervisor ------------------------------------------------------------------------- Signature ------------------------------------------------- Date ------------------------------ Department of Chemical Engineering Caritas University Amorji-Nike Enugu DEDICATION This project was dedicated to Jesus the Saviour for all his marvelous deeds in my life, more especially for seeing me through all my university years and to my dearly parents Mr. and Mrs. Adike, N. Joseph, for all their contributions, encouragement, love, understanding and care for me and the family. ACKNOWLEDGEMENT To Jesus the Saviour be the glory to whom I depend on I wish to thank the Almighty God for giving me the opportunity, knowledge and wisdom to under take my university task successfully. My profound gratitude goes to my supervisor, Engr. (Mr.) Ken Ezeh for is invaluable supervision and unremitting attention. I wish also to acknowledge my lecturers in the person of Engr. Prof. J.I. -

Croconate Salts. New Bond-Delocalized Dianions, &Q
JOURNAL OF RESEARCH of the National Bureau of Standards Volume 85, No.2, March·April1980 Pseudo-Oxocarbons. Synthesis of 2, 1,3-Bis-, and 1, 2, 3-Tris (Dicyanomethylene) Croconate Salts. New Bond-Delocalized Dianions, "Croconate Violet" and "Croconate Blue"* Alexander J. Fatiadit National Bureau of Standards, Washington, D.C. 20234 October 24,1979 Synthesis and characteri zation of new bond·delocalized dianions, e.g., 2, 1,3·bis·, 1,2, 3·tris (di cyanomethyl. ene) croconate salts have been described. The dianions re ported represent a new class of aromati c, nonbenze· noid co mpounds, named pseudo·oxocarbons. A study of their physical, analytical and chemical properties offer a new direction in the chemistry of oxocarbons. Key words: Acid; aromatic; bond·delocalized; croco nic; diani on; malononitrile; nonbenzenoid; oxocarbon; salt; synthesis 1. Introduction molecular properties of the croconic salts (e.g. 2 , dipotas sium salt) were first seriously investigated when a symmetri The bright ye ll ow dipotassium croconate 1 and croconic cal, delocalized structure fo r the dianion 2 was proposed by acid (1 , K = H, 4,5-dihydroxy-4--cyclopentene-l,2,3-trione) Yamada et aJ. [3] in 1958. A few years later [4], the d i anion 2 were first isolated by Gmelin [1]' in 1825, from the black, ex· and the related deltate [5], squarate, rhodizonate, and plosive, side-reaction product (e.g. K6 C6 0 6 + KOC=COK), tetrahydroxyquinone anions were recognized by West et aJ. by the reaction of carbon with potassium hydroxide, in a [2,4] as members of a new class of aromatic oxocarbons pioneer, industrial attempt to manufacture potassium. -

Graphether:A Two-Dimensional Oxocarbon As a Direct Wide-Gap Semiconductor with High Mechanical and Electrical Performances
Graphether:A Two-Dimensional Oxocarbon as a Direct Wide-Gap Semiconductor with High Mechanical and Electrical Performances Gui-Lin Zhu1‖, Xiao-Juan Ye1,2‖, and Chun-Sheng Liu1,2* 1Key Laboratory of Radio Frequency and Micro-Nano Electronics of Jiangsu Province, College of Electronic and Optical Engineering, Nanjing University of Posts and Telecommunications, Nanjing 210023, China 2School of Engineering, University of British Columbia, Kelowna, BC V1V 1V7, Canada ‖The first two authors contributed equally to this work. *E-mail: [email protected] Abstract: Although many graphene derivatives have sizable band gaps, their electrical or mechanical properties are significantly degraded due to the low degree of π-conjugation. Besides the π-π conjugation, there exists hyperconjugation interactions arising from the delocalization of σ electrons. Inspired by the structural characteristics of a hyperconjugated molecule, dimethyl ether, we design a two-dimensional oxocarbon (named graphether) by the assembly of dimethyl ether molecules. Our first-principle calculations reveal the following findings: (1) Monolayer graphether possesses excellent dynamical and thermal stabilities as demonstrated by its favourable cohesive energy, absence of the soft phonon modes, and high melting point. (2) It has a direct wide-band-gap of 2.39 eV, indicating its potential applications in ultraviolet optoelectronic devices. Interestingly, the direct band gap feature is rather robust against the external strains (-10% to 10%) and stacking configurations. (3) Due to the hyperconjugative effect, graphether has the high intrinsic electron mobility. More importantly, its in-plane stiffness (459.8 N m-1) is even larger than that of 1 graphene. (4) The Pt(100) surface exhibits high catalytic activity for the dehydrogenation of dimethyl ether. -

Generation and Characterization of Hardwood Smoke Inhalation Exposure Atmospheres
Aerosol Science and Technology ISSN: 0278-6826 (Print) 1521-7388 (Online) Journal homepage: https://www.tandfonline.com/loi/uast20 Generation and Characterization of Hardwood Smoke Inhalation Exposure Atmospheres Jacob D. McDonald , Richard K. White , Edward B. Barr , Barbara Zielinska , Judith C. Chow & Eric Grosjean To cite this article: Jacob D. McDonald , Richard K. White , Edward B. Barr , Barbara Zielinska , Judith C. Chow & Eric Grosjean (2006) Generation and Characterization of Hardwood Smoke Inhalation Exposure Atmospheres, Aerosol Science and Technology, 40:8, 573-584, DOI: 10.1080/02786820600724378 To link to this article: https://doi.org/10.1080/02786820600724378 Published online: 01 Feb 2007. Submit your article to this journal Article views: 355 Citing articles: 17 View citing articles Full Terms & Conditions of access and use can be found at https://www.tandfonline.com/action/journalInformation?journalCode=uast20 Aerosol Science and Technology, 40:573–584, 2006 Copyright c American Association for Aerosol Research ISSN: 0278-6826 print / 1521-7388 online DOI: 10.1080/02786820600724378 Generation and Characterization of Hardwood Smoke Inhalation Exposure Atmospheres Jacob D. McDonald,1 Richard K. White,1 Edward B. Barr,1 Barbara Zielinska,2 Judith C. Chow,2 and Eric Grosjean3 1Lovelace Respiratory Research Institute, Albuquerque, New Mexico, USA 2Desert Research Institute, Reno, Nevada, USA 3Daniel Grosjean and Associates, Inc., Ventura, California, USA INTRODUCTION An inhalation exposure system and operating protocol were de- The National Environmental Respiratory Center (NERC) was veloped that resulted in consistent achievement of targeted expo- established to conduct laboratory research that improves our un- sure concentrations of hardwood smoke without compromising the derstanding of the contributions of individual air contaminants desire to include multiple phases of a burn cycle. -

Conceptual Progress for Explaining and Predicting Self-Organization on Anodized Aluminum Surfaces
nanomaterials Review Conceptual Progress for Explaining and Predicting Self-Organization on Anodized Aluminum Surfaces Mikhail Pashchanka Department of Chemistry, Eduard-Zintl-Institute, Technical University of Darmstadt, Alarich-Weiss-Straße 12, 64287 Darmstadt, Germany; [email protected] Abstract: Over the past few years, researchers have made numerous breakthroughs in the field of aluminum anodizing and faced the problem of the lack of adequate theoretical models for the interpretation of some new experimental findings. For instance, spontaneously formed anodic alumina nanofibers and petal-like patterns, flower-like structures observed under AC anodizing conditions, and hierarchical pores whose diameters range from several nanometers to sub-millimeters could be explained neither by the classical field-assisted dissolution theory nor by the plastic flow model. In addition, difficulties arose in explaining the basic indicators of porous film growth, such as the nonlinear current–voltage characteristics of electrochemical cells or the evolution of hexagonal pore patterns at the early stages of anodizing experiments. Such a conceptual crisis resulted in new multidisciplinary investigations and the development of novel theoretical models, whose evolution is discussed at length in this review work. The particular focus of this paper is on the recently developed electroconvection-based theories that allowed making truly remarkable advances in understanding the porous anodic alumina formation process in the last 15 years. Some explanation of the synergy between electrode reactions and transport processes leading to self-organization is provided. Finally, future prospects for the synthesis of novel anodic architectures are discussed. Citation: Pashchanka, M. Conceptual Progress for Explaining Keywords: porous anodic alumina (PAA); chaos and self-organization theory; electroconvection; and Predicting Self-Organization on colloidal gel model; anion exchange; DLVO theory; fluid mechanics; surface chemistry; surface Anodized Aluminum Surfaces. -

Carbon Monoxide Poisoning
CONTINUING MEDICAL EDUCATION: SITARAM SHRESTHA – CARBON MONOXIDE POISONING Carbon monoxide poisoning Sitaram Shrestha Professor, Department of General Practice and Emergency Medicine, Patan Academy of Health Sciences, Lalitpur, Nepal ABSTRACT Carbon monoxide (CO) poisoning may be suicidal, homicidal and in many cases it is accidental. Poisoning is due to formation of carboxyhaemoglobin, which interferes transfers of oxygen and leads to appearance of symptoms.1 Which are not specific and may similar to COVID19. Symptoms depends upon concentration of CO in surroundings. Antidote of CO is oxygen. Keywords: carbon monoxide, poisoning, toxicity CORRESPONDENCE Dr. Sitaram Shrestha Department of General Practice and Emergency Medicine, Patan Academy of Health Sciences, Lalitpur, Nepal Email: [email protected] Journal of General Practice and Emergency Medicine of Nepal. Issue 10: 2021. Available at: www.jgpeman.com, eISSN: 2363-1168 55 CONTINUING MEDICAL EDUCATION: SITARAM SHRESTHA – CARBON MONOXIDE POISONING INTRODUCTION • Low blood pressure Carbon monoxide (CO) Is known since ancient time • Muscle weakness which is tasteless, colour less, odour less and • Rapid or abnormal heartbeat non-irritating gas. It is sometimes termed a silent • Shock killer. As the specific gravity of CO is 0.97, it is • Nausea and vomiting slightly lighter than air. This gas is mainly produced • Unconsciousness by incomplete combustion of organic compounds.2 • Delayed poisoning Carbon monoxide consists of one carbon atom and one oxygen atom, connected by a triple -

ABSTRACT LI, SHULI. Preparation and Integration of Composite Nanofiber Anodes in Advanced Lithium-Ion Batteries
ABSTRACT LI, SHULI. Preparation and Integration of Composite Nanofiber Anodes in Advanced Lithium-Ion Batteries. (Under the direction of Dr.Xiangwu Zhang). Lithium-ion batteries are receiving considerable attention for use in portable electronics, electric vehicles and large-scale smart grids due to their superior energy storage capability over other rechargeable batteries. To achieve super-high energy density, advanced electrode materials with high energy storage capacities must be developed to replace traditional LiCoO2 cathode and graphite anode materials. For cathode materials, lithium metal oxides with a layered structure or a spinel structure, polyanion-based compounds and special organic materials have been investigated and reported in the past few years. They show inherent advantages of good structure stability, low cost, and high rate capability, etc. For anode materials, carbonaceous materials, lithium alloys and transition metal oxides are among the most promising alternatives and dominate current research. They show intriguing advantages of good environment compatibility, large specific capacities, and high safety, etc. Anode materials with capacities far beyond that of graphite are recently studied in order to satisfy the continuous demands for lithium-ion batteries that power advanced electronic devices. Among various types of anode materials with different elements and structures, carbon nanofiberous materials with controllable surface areas have attracted significant attention in recent years and are considered to be promising candidates for high-performance electrode materials. Carbon nanofibers can be produced by many techniques, among which electrospinning is a particularly efficient, simple and inexpensive method. In this work, we focused on fabricating various nanoparticle-loaded carbon nanofiber composite anodes and explore their potential applications as anode materials for advanced lithium-ion batteries. -

AIM and NBO Analysis of Oxocarbon Heterocumulenes As Divalent Carbon(0) Species Final Paper
Massachusetts Institute of Technology 5.05 Main Group Chemistry AIM and NBO Analysis of Oxocarbon Heterocumulenes as Divalent Carbon(0) Species Final Paper Author: Jonathan Melville Instructor: Christopher C. \Kit" Cummins December 13, 2016 Contents 1 Abstract 1 2 Introduction 1 3 Results and Discussion 1 3.1 Dicarbon Monoxide (C2O)..........................1 3.1.1 C2O..................................2 3.1.2 C2O·BH3 ...............................2 3.1.3 C2O·2 BH3 ..............................3 3.1.4 C2O·3 BH3 ..............................4 3.2 Carbon Suboxide (C3O2)...........................5 3.2.1 C3O2 ..................................6 3.2.2 C3O2·BH3 ...............................7 3.2.3 C3O2·2 BH3 ..............................8 4 Experimental 9 5 Conclusion 9 6 Acknowledgments 10 References 10 List of Figures 1 NRT-calculated C2O resonance structures . .2 2 C2O geometry . .2 3 C2O·BH3 geometry . .2 4 NRT-calculated C2O·BH3 resonance structures . .3 5 C2O·2 BH3 geometry . .3 6 NRT-calculated C2O·2 BH3 resonance structures . .4 7 NRT-calculated C2O·3 BH3 resonance structures . .5 8 C2O·3 BH3 geometry . .5 9 NRT-calculated C3O2 resonance structures . .6 10 C3O2 geometry . .6 11 C3O2·BH3 geometry . .7 12 NRT-calculated C3O2·BH3 resonance structures . .7 13 C3O2·2 BH3 geometry . .8 14 NRT-calculated C3O2·2 BH3 resonance structures . .9 List of Tables 1 NRT resonance state weightings of C2O. .2 2 Selected NRT resonance state weightings of C2O·BH3............3 3 Selected NRT resonance state weightings of C2O·2 BH3...........4 4 Selected -

Anomalous Mechanical Behavior of the Deltic, Squaric and Croconic Cyclic Oxocarbon Acids
IOP Publishing Journal Title Journal XX (XXXX) XXXXXX https://doi.org/XXXX/XXXX Anomalous mechanical behavior of the deltic, squaric and croconic cyclic oxocarbon acids Francisco Colmenero1 1 Molecular Physics Department, Instituto de Estructura de la Materia (CSIC), Madrid, Spain E-mail: [email protected] Received xxxxxx Accepted for publication xxxxxx Published xxxxxx Abstract The structural and mechanical properties of the deltic, squaric and croconic cyclic oxocarbon acids were obtained using theoretical solid-state methods based in Density Functional Theory employing very demanding calculation parameters in order to yield realistic theoretical descriptions of these materials. The computed lattice parameters, bond distances, angles, and X-ray powder diffraction patterns of these materials were in excellent agreement with their experimental counterparts. The crystal structures of these materials were found to be mechanically stable since the calculated stiffness tensors satisfy the Born mechanical stability conditions. Furthermore, the values of the bulk modulus and their pressure derivatives, shear and Young moduli, Poisson ratio, ductility and hardness indices, as well as mechanical anisotropy measures of these materials were reported. A complete review of the literature concerning the negative Poisson ratio and negative linear compressibility phenomena is given together with the theoretical study of the mechanical behavior of cyclic oxocarbon acid materials. The deltic, squaric, and croconic acids in the solid state are highly anisotropic materials characterized by low hardness and relatively low bulk moduli. The three materials display small negative Poisson ratios. The croconic acid displays the phenomenon of negative linear compressibility for applied pressures larger than ~0.4 GPa directed along the direction of minimum Poisson ratio and undergoes a pressure induced phase transition at applied pressures larger than ~1.0 GPa.