The Chemical Composition of Maple Syrup
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Maple Sugar Blondies
S H E L B U R N E F A R M S Maple Sugar Blondies Adapted from Cooking with Shelburne Farms by Melissa Pasanen with Rick Gencarelli INGREDIENTS For the dough 2¼ cups all-purpose flour 1 teaspoon baking soda ½ teaspoon salt ¾ cup canola oil ¼ cup Vermont maple syrup (use Grade A: Dark Color with Robust Taste or Grade A: Very Dark Color with Strong Taste for a stronger maple flavor) ¾ cup granulated maple sugar (or packed light brown sugar) 1 large egg beaten For the frosting ¼ cup Vermont maple syrup (see note above) ½ cup granulated maple sugar ½ cup confectioner’s sugar 6 tablespoons cold, unsalted butter cut into small pieces ½ teaspoon pure vanilla extract PREPARATION 1. Preheat the oven to 350°F. Lightly grease a 9x13-inch baking pan. In a medium bowl, whisk together the flour, baking soda, and salt. 2. In a separate bowl, beat together the canola oil, maple syrup, maple sugar, and white sugar until well blended. (If you have one, use a stand mixer fitted with a paddle attachment on medium speed.) Add the egg in a slow stream. Mix in the flour mixture in thirds, blending after each addition. (Use low speed in stand mixer.) 3. Press dough evenly into prepared pan. Bake 20-25 minutes until the blondies are golden brown and starting to crack on the top like brownies. Remove the pan to a cooling rack and cool for about 30 minutes before frosting. It should be warm to the touch, not hot. 4. The frosting: While the blondies are baking, bring the maple syrup to a simmer in a medium, heavy-bottomed saucepan over medium-high heat. -

Can United States Sugar Maple (Acer Saccharum) Syrup Production Be Maintained in a Warming Climate? Stephen N
INTERNATIONAL JOURNAL OF BIODIVERSITY SCIENCE, ECOSYSTEM SERVICES & MANAGEMENT, 2017 VOL. 13, NO. 2, 40–52 http://dx.doi.org/10.1080/21513732.2017.1285815 Special Issue: Ecosystem Services Nexus Thinking Managing for delicious ecosystem service under climate change: can United States sugar maple (Acer saccharum) syrup production be maintained in a warming climate? Stephen N. Matthewsa,b and Louis R. Iverson b aSchool of Environment and Natural Resources, Ohio State University, Columbus, OH, USA; bNorthern Research Station, USDA Forest Service, Delaware, OH, USA ABSTRACT ARTICLE HISTORY Sugar maple (Acer saccharum) is a highly valued tree in United States (US) and Canada, and Received 9 June 2016 its sap when collected from taps and concentrated, makes a delicious syrup. Understanding Accepted 18 December 2016 how this resource may be impacted by climate change and other threats is essential to EDITED BY continue management for maple syrup into the future. Here, we evaluate the current Christine Fürst distribution of maple syrup production across twenty-three states within the US and estimate the current potential sugar maple resource based on tree inventory data. We KEYWORDS model and project the potential habitat responses of sugar maple using a species distribu- Maple syrup; climate tion model with climate change under two future General Circulation Models (GCM) and change; sugar maple (Acer saccharum emission scenarios and three time periods (2040, 2070, 2100). Our results show that under ); ecosystem services; Eastern United GFDL-A1Fi (high CO2 emissions), sugar maple habitat is projected to decline (mean ratio of States future habitat to current habitat per state = 0.46, sd ± 0.33), which could lead to reduced maple syrup production per tree and nearly 5 million additional taps required to maintain current projection levels. -

Replacing Table Sugar with Maple Sugar by STEPHEN CHILDS
Cornell Maple Bulletin 205 (2007) Replacing Table Sugar with Maple Sugar by STEPHEN CHILDS Balancing Balancing ingredients Ingredients Replacing granulated cane or beet sugar in recipes with maple syrup should be a growing trend. Guidelines about sugar replacement are different in different sources. It is easy to understand this confusing situation when you realize there are actually two ingredients that need to be balanced. When replacing granulated sugar in a recipe with maple syrup you should consider both the sugar balance and the liquid balance of the recipe. Some recommendations say to add 1 ! cup of syrup to replace one cup of sugar, others say to replace one cup of sugar with " cup of maple syrup. One is trying to balance the liquid in the recipe, the other the sweetness. The most straightforward approach is to simply replace one cup of granulated cane sugar with one cup of granulated maple sugar. In this case you gain the extra flavors from maple while the sweetness and the liquid stay in balance. I would especially suggest this where the recipe is depending on the qualities of milk or another liquid that you may be reducing to perform some important function in the recipe beyond what simply using water would accomplish. Liquid vs. Dry Liquid vs. Dry One cup of maple syrup at a fairly common density of 67º Brix provides 7.5 ounces (214 grams) of sugar and 3.7 ounces (105 grams) of water. One cup of cane sugar averages about 7.4 ounces (210 grams) of sugar. This is roughly the same amount sugar in a cup of maple syrup as in a cup of granulated sugar. -
Determination of Glucose, F R U C T O S E, and Sucrose in Maple Syrup
ADVERTISING SUPPLEMENT Food and Bevera g e 5 3 THE A P P L I C AT I O N NOTEBOOK — June 2005 Determination of Glucose, F r u c t o s e, and Sucrose in Maple Syrup Randy Benton, M e t rohm-Peak, Inc aple syrup is produced by boiling the sap from va r i o u s Results and Discussion M types of maple trees. Ge n e r a l l y, it is produced in the With the adulteration of maple syrup as a growing concern a sim- No rtheastern United States and Canada in late winter or early ple yet rugged method is needed to determine if syrup has been spring when the sap of the tree is running. Many other pro d u c t s a l t e red. Larger peak area and concentration values are expected in such as maple sugar, maple butter (cream), maple candies, and more s y r up that has been altered. For this study, samples of maple syru p a re also produced using the sap from maple trees. One issue pro- we re diluted, fil t e red, and then injected for analysis. A sample of d u c e r’s face is that of adulteration of the syrup by the addition of u n a l t e red maple syrup was spiked with high-fructose corn syrup to either corn syrup or cane sugar. A small amount of either of the two determine if a change would be seen. -

Cornell Maple Program
Cornell Maple Program 2019 – 2020 ANNUAL REPORT Cornell University Department of Natural Resources Authored by: Stephen Childs, Ailis Clyne, Aaron Wightman and Adam Wild 1 Program Overview Supporting the Maple Products Industry The Cornell Maple Program conducts research and extension with the goal of improving the production and use of maple products. Work toward this objective takes place in three parts: 1) infrastructure upgrades that create and enhance capacity in our two maple facilities 2) applied research on a broad array of topics related to maple production and profitability and, 3) extension programming to share knowledge across the industry. The Cornell Maple Program consists of maple specialists and technical support staff located at two facilities who work with a network of industry allies to deliver educational content. The Arnot Research Sugarbush near Ithaca, NY and the Uihlein Maple Research Forest in Lake Placid, NY include a combined capacity of over 14,000 taps in 350 acres of sugarbush, as well as modern processing equipment and research kitchen space. These two research and production facilities provide opportunities for experiments that account for region-wide variability in sugarbush conditions. Research sugarhouses at Arnot Forest in Van Etten, NY (left) and Uihelin Forest in Lake Placid, NY (right). 2 Program Highlights Sugarbush Management Super Sweet Trees Continuing the legacy of the “super sweet” maple tree project at the Uihlein Research Forest, we are in the middle of a three year project funded by McIntire Stennis federal capacity funds to re-analyze the potential sap sweetness and volume from our “super sweet” maple plantations. -

The Sugar Maple Acer Saccharum
The Sugar Maple Acer Saccharum Cris Soliman & Hanna Barnes OEB59-- Plants and Human Affairs Botanical information The sugar maple is one of 148 maple species found in North America, of which 90 are native. The sugar maple is notable for the following 1 characteristics : ● Mature trees range in height from 70-90 feet, with an average diameter of 2-3 feet. ● Leaves are simple and single, with five lobes. The boundaries between the lobes are smooth and shallow (1a), helping to distinguish it from the red maple. ● Leaves and buds are in an opposite arrangement (1d). ● Terminal buds are typically larger than lateral buds (1d). ● Twigs are covered in lenticels-- small openings in the bark. ● Mature bark appears to have ‘plates’ that peel along the long edge. ● Monoecious-- female and male flowers on the same plant (1b, c). ● Fruit is a double samara (1e), meaning it has a characteristic winged shape. ● Massive quantities of fruits are produced cyclically, usually every two to five years, after the tree matures at age thirty. ● Small pores that are uniformly spaced (diffuse-porous). Distribution: ● From Nova Scotia and Quebec in the northeast, west to Minnesota and south to Tennessee. 1. Luzadis, V.A. & Gossett, E.R. (1996). Sugar Maple. In J.P. Lassoie, V.A. Luzadis, and D.W. Grover (Eds.), Forest Trees of the Northeast (157-166). Ithaca, NY: Cornell Cooperative Extension. What is sugar maple sap?3 ● The maple sap is the vehicle which transports nutrients Sugar Maple Products through the xylem. ● Sap is a solution of sugar-- mostly sucrose-- in water. -

1 Explore January: the History of Maple Sugaring Learn How Maple
Explore January: The History of Maple Sugaring Learn how maple sap used to be collected and processed and how it compares to current techniques. Maple sugaring is the method of making maple syrup from sap. Humans have been collecting sap to make syrup for many years. Evidence shows that sap collection and production may have even started before or around the beginning of the 17th century. In the earlier years, some Indigenous peoples collected sap from sugar maple trees and hollowed out wooden logs to create troughs. These would act as a pot for the sap to go into and then stones were heated in a fire and placed inside the trough to boil. By boiling down the tree sap, the water evaporated out as steam which therefore condensed the liquid down into an edible A trough with hot rocks to boil down sap. thick, sweet, sticky syrup product. There were at least three different forms of sugar that were then produced from maple syrup: 1. Grain sugar – similar to today’s brown sugar. 2. Cake sugar – like maple candy, it is poured into molds and left to harden for easy, long-term storage. 3. Wax sugar – also known now as “sugar on snow”, maple sugar is heated and poured over shaved ice. The Importance of Maple Trees Maple trees and sap were important to many Indigenous peoples and various tribes have legends that highlight this aspect. For example, the Abenaki are one of the Algonquian-speaking peoples of northeastern North America. The Abenaki originated in a region called Wabanahkik in the Eastern Algonquian languages (meaning "Dawn Land"), a territory now including parts of Quebec and the Maritimes of Canada and northern sections of the New England region of the United States. -

Naturally Sweet
A FAMILY TRADITION ALL NATURAL PRODUCT NATURALLYNATURALLYNATURALLY SWEETSWEETSWEET CATALOG Pure Maple Syrup Since 1928 A Labor Of Love As the snow begins to melt in Northwestern Wisconsin, we make our way into the sugarbush to collect what the Native Americans called “Sweet Water” from the sugar maples. In the spring of the year, sweet sap is flowing from the tree, so we spend long days collecting every drop. Throughout the night, we boil the sap down to Pure Maple Syrup, then the syrup is boiled once again just before bottling to “lock-in” the freshness that is Anderson’s Pure Maple Syrup. Throughout the pages of this brochure, you will find the fruits of our labor. Pure Maple Syrup products in all shapes and sizes. All of them have one thing in common, they are the result From the tree... of our family’s 3 generation passion for producing the best Pure Maple Syrup. To the cooker ... To YOU. Pure Maple Syrup Glass bottles are our most popular containers and can be found in grocery stores across the United States. The glass helps maintain our syrup’s fresh flavor longer and shows off it’s beautiful amber glow. Economy Glass Bottles Ounces Size Case Pack Item # 8 oz. ½ Pint 12 12008C 12 oz. ¾ Pint 12 12012C 16 oz. Pint 12 12017C 32 oz. Quart 6 12033C Organic Pure Maple Syrup Our Certified Organic Pure Maple Syrup is produced according to strict organic guidelines and inspections. The result is Pure Maple Syrup that’s as great tasting and natural as nature intended. -

SOIL NUTRIENTS AFFECT SWEETNESS of SUGAR MAPLE SAP by Adam D. Wild a Thesis Submitted in Partial Fulfillment of the Requiremen
SOIL NUTRIENTS AFFECT SWEETNESS OF SUGAR MAPLE SAP by Adam D. Wild A thesis submitted in partial fulfillment of the requirements for the Masters of Science Degree State University of New York College of Environmental Science and Forestry Syracuse, New York April 2014 Approved: Department of Forest and Natural Resources Management Ruth D. Yanai, Major Professor Timothy Toland, Chair Examining Committee David Newman, Department Chair S. Scott Shannon, Dean The Graduate School © 2014 Copyright Adam D. Wild All rights reserved Acknowledgements This study would not have been possible without the assistance of my advisor Ruth Yanai who diligently worked with me and provided the opportunity to explore my own interest. My graduate committee members Chris Nowak, Colin Beier and Mike Farrell were always willing to provide assistance. Acknowledgement of Eric Randall for his advice on sap sampling is necessary along with Tim Wilmot, Tim Perkins, Steve Childs, Paul Schaberg, Heidi Asbjornsen, Adan Hernádez, and Sandy Wilmot for their many contributions. Mike Wild willing helped during the last sap sampling period which allowed for a shorter day in the field. Gas exchange measurements could not have been taken without Katherine Sinacore operating the portable photosynthesis system. Chris Costello was always willing to assist me with the snowmobile for sap sampling and ensure I came out of the woods safely. Melany Fisk was gracious in providing the soils data. Members of my lab, Craig See, Franklin Diggs, Yang Yang and Yi Dong were always willing to provide feedback on results. Foliage and tree growth sampling was completed by members of the MELNHE summer field crew including Eric MacPherson who assisted with the canopy assessment. -
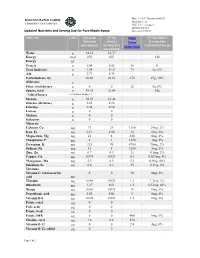
Updated Nutrients and Serving Size for Pure Maple Syrup: Daily Value
Date: 1/15/17 Document #1017 BASCOM MAPLE FARMS Revision #: A COMPANY DOCUMENT SQF 2.3.4 (2 pages) Revised 9/25/18 Updated Nutrients and Serving Size for Pure Maple Syrup: Reviewed 8/26/19 Nutrient Units 100 gram 39.78g Daily 39.78g (30mL) Nutrients (30mL) Value Serving Size (unrounded) Serving Size (DRV/RDI) (Nutritional Facts) (unrounded) Water g 32.15 12.79 Energy kcal 270 107 110 Energy kJ Protein g 0.04 0.02 50 0 Total lipid (fat) g 0.24 0.10 78 0g, 0% Ash g 0.71 0.28 Carbohydrate, by 66.89 26.61 275 27g, 10% difference g Fiber, total dietary g 0 0 28 0g, 0% Sugars, total g 59.92 23.84 24g *Added Sugars see bottom of page 2 Sucrose g 58.93 23.44 Glucose (dextrose) g 0.65 0.26 Fructose g 0.34 0.14 Lactose g 0 0 Maltose g 0 0 Galactose g 0 0 Minerals Calcium, Ca mg 73 29 1300 29mg, 2% Iron, Fe mg 0.11 0.04 18 0mg, 0% Magnesium, Mg mg 21 8 420 8mg, 2% Phosphorus, P mg 2 1 1250 1mg, 0% Potassium, K mg 225 90 4700 90mg, 2% Sodium, Na mg 12 5 2300 5mg, 0% Zinc, Zn mg 0.7 0.3 11 0.3mg, 2% Copper, Cu mg 0.074 0.029 0.9 0.029mg, 4% Manganese, Mn mg 2.3 0.9 2.3 0.9mg, 40% Selenium, Se µg 0.6 0.2 55 0.2mg, 0% Vitamins Vitamin C, total ascorbic 0 0 90 0mg, 0% acid mg Thiamin mg 0.066 0.026 1.2 1.2mg, 2% Riboflavin mg 1.27 0.51 1.3 0.51mg, 40% Niacin mg 0.081 0.032 16 0mg, 0% Pantothenic acid mg 0.03 0.01 5 0mg, 0% Vitamin B-6 mg 0.002 0.001 1.7 0mg, 0% Folate, total µg 0 0 Folic acid µg 0 0 Folate, food µg 0 0 Folate, DFE µg 0 0 400 0mg, 0% Choline, total mg 1.6 0.6 550 Vitamin B-12 µg 0 0 2.4 0µg, 0% Vitamin B-12, added µg 0 0 Page 1 of 2 Vitamin -

Sugar Maple Acer Saccharum
sugar maple Acer saccharum Kingdom: Plantae Division/Phylum: Magnoliophyta Class: Magnoliopsida Order: Sapindales Family: Aceraceae ILLINOIS STATUS common, native FEATURES The deciduous sugar, or hard, maple tree may grow to a height of 80 feet and a trunk diameter of three feet. Its bark may be gray, dark brown or black, becoming furrowed and scaly as it ages. The simple leaves are arranged oppositely along the stem. Each leaf may grow to five inches long and at least that wide. The leaf has three to five coarsely toothed lobes. The upper surface of the leaf is dark green and smooth while the lower surface is paler and smooth. Leaves turn yellow or orange in the fall. Male (staminate) and female (pistillate) flowers are separate. They may or may not be located on the same tree. The small yellow-green flowers are borne in clusters. Fruits are in pairs and consist of a wing with a seed at the base. The seed is yellow-green to brown, about one inch long. BEHAVIORS The sugar maple may be found statewide in Illinois. This tree grows in moist woods. It flowers in April and May as its leaves begin to unfold. The heavy, strong wood is used for making furniture, for interior finishing and in constructing cabinets. Maple sugar is derived from its sap. This tree is often grown as an ornamental. HABITATS Aquatic Habitats bottomland forests Woodland Habitats bottomland forests; upland deciduous forests Prairie and Edge Habitats none © Illinois Department of Natural Resources. 2017. Biodiversity of Illinois. . -

L845 Trees and Shrubs That Attract Songbirds and Wildlife
Trees and Shrubs That Attract Songbirds and Wildlife Food Plant Name Quality Season Cover Nesting Range* Plant Habitat Remarks Evergreen Trees Juniper, Rocky Mountain Good Fall- Excellent Excellent W Average Similar to eastern redcedar; (Juniperus scopulorum) Winter conditions birds eat seeds; dense foliage used by wildlife Pine, Eastern White Fair Fall Good Good E Best on moist, Keep away from exposed sites; (Pinus strobus) well-drained, seeds eaten by birds and small acidic soil mammals Pine, Ponderosa Good Fall Good Good C,W Average Very drought tolerant; seeds (Pinus ponderosa) conditions eaten by numerous birds and small mammals and used for cover Pine, Southwestern Fair Fall Good Good E,C,W Average Cover for various wildlife; White conditions seeds eaten by birds and small (Pinus strobiformis) mammals Redcedar, Eastern Good Fall- Excellent Excellent E,C,W Average Do not use near apples or crab (Juniperus virginiana) Winter conditions apples; birds eat seeds; dense foliage used by wildlife Spruce, Black Hills Fair Fall Good Good E,C Average Slow growing; provides habitat (Picea glauca var. densata) conditions; for songbirds prefers acid soil Deciduous Trees Baldcypress Poor Summer- Good Good E Generally wet soil, Resting and nesting cover for (Taxodium distichum) Fall but will tolerate many species of wildlife dry site; not for alkaline soil Basswood Good Fall- Good Good E Prefers deep, well- Great for honeybees; forms (Tilia americana) Winter drained soils cavities when older Blackgum Good Fall Good Good E Moist, deep, Fruit eaten