Development of an Universal Paint Stripper for Metals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Wood Preparation
WOOD PREPARATION Penofin® Pro-Tech Stripper pecially developed to effectively remove oil • Fast acting Sfinishes on all exterior wood, composite or concrete surfaces using New Clean Strip Technology. • Easy to use Pro-Tech Stripper leaves little or no residue, will not • Removes paint, stains, sealers raise grain and provides double the coverage of average strippers. Pro-Tech Stripper is first step in preparing and loose wood fibers your wood for a beautiful Penofin application • Certified no VOC’s product Penofin® Pro-Tech Cleaner he best outdoor cleaner for just about everything, • Restores wood to mill Tthis unique formula uses Super Hydrogen Power to tackle just about any cleaning job around bright appearance your home. Mix these concentrated granules in • Cleans and rejuvenates wood varying strengths to effectively remove grease, grime, dirt, organic stains, tree sap and mineral deposits. • Easy to apply Kills mold and mildew. For use on wood, masonry, • Biodegradable; safe on soil concrete, fiber cement, fiberglass, outdoor furniture, and plant life cushions and floor coverings, glass and tile. Penofin® Pro-Tech Brightener nnihilates tough tannin stains and watermarks • The hardwood helper Aon all types of wood decking siding and fences; removes mill glaze. Penofin Pro-Tech Brightener • Reduces mill glaze for reestablishes the Ph balance of your wood after better penetration stripping and cleaning and brightens gray weathered • Removes the gray wood to bring back that mill-bright color. • Prepares hardwood for finishing Materials and tools for the project Before 16 WOOD PREPARATION WOOD PREPARATION BEFORE FINISHING Cleaning and preparing wood surface. There are variables to consider when preparing and finishing a Old wood: Old wood is more porous and may need more wood surface is the wood new, old or weathered? stain to cover the surface. -

Installation, Care, and Maintenance of Wood Shake and Shingle Siding
United States Department of Agriculture Installation, Care, and Forest Service Maintenance of Wood Forest Products Laboratory Shake and Shingle Siding General Jack Dwyer Technical Report Tony Bonura FPL–GTR–202 Arnie Nebelsick Sam Williams Christopher G. Hunt Abstract Contents This article gives general guidelines for selection, instal- Introduction ......................................................................... 1 lation, finishing, and maintenance of wood shakes and Selection .............................................................................. 1 shingles. The authors gathered information from a variety of Shakes ............................................................................. 1 sources: research publications on wood finishing, technical data sheets from paint manufacturers, installation instruc- Shingles ........................................................................... 2 tions for shake and shingle siding, and interviews with Specialty Sidewall Products ............................................ 3 experts having experience constructing and inspecting shake Installation ........................................................................... 5 and shingle siding. If research reports could not be found, the recommendations are based on opinions of experts and Rain-Screen Method ....................................................... 5 practices that have been shown to give good service life for Direct Application ........................................................... 6 shakes and shingles. -

Winter 2021 Digital Boomer
HEARTH & HOME ASK AMY HEALTH & WELLNESS Decorating & Selling DNA Disasters Healthy, Legal Mushrooms WINTER 2020 Virginia’sGUITAR MAKERS Master FROM OUR READERS Car Collector Childhood & Candy Family Pool Table TRAVEL Kentucky Bourbon Country Joseph Rosendo’s Travel Musings Travel Insurance Museum of the U.S. Army Fredericksburg, Texas Nostalgia • Food & Booze Plus Books • Giving Back Fun & Games CONTENTS Vol. 15 , No. 4 WINTER ’20 HEARTH & HOME ASK AMY Decorating & Selling HEALTH & WELLNESS DNA Disasters Healthy, Legal Mushrooms THE CREATIVE LIFE WINTER 2020 2 Virginia’s Master Guitar Makers ON THE 4 Behind the Scenes with Art Conservators Virginia’s Master COVER GUITAR MAKERS J. PlunkyFROM Branch OUR READERS This custom guitar was FROM OUR Photograph by READERS 5 Confessions of a Car Collector Car Collector Patrick Mamou made by the craftsmen Childhood & Candy 6 Memories of Childhood and Candy Family Pool Table TRAVEL at Rockbridge Guitar Co. Kentucky Bourbon Country 7 The Family Pool Table Joseph Rosendo’s Travel Musings Travel Insurance Museum of the U.S. Army in Charlottesville, Virginia. Fredericksburg, Texas Nostalgia • Food & Booze Plus Books • Giving Back YESTERYEAR Fun & Games 8 Jacqueline Bisset’s Sizzling Career Photograph by JJ Huckin 9 ‘Tinker Bell’ Model Engages with Flame TRAVEL 10 Experience Kentucky Bourbon Country FOOD, BREWS, & BOOZE 11 Joseph Rosendo, Cultivating Memories from Travel Experiences 24 Virginia Recipes for Home Cooks 12 Fredericksburg, Texas: A Tantalizing Twist 25 Explore American Craft Beer from Home -

SK HD Paint Stripper.Indd
Heavy Duty Paint Stripper multi-layer paint remover DESCRIPTION AND USE Limitations Sure Klean® Heavy Duty Paint Stripper, an alkaline formula with •Product effi ciency is reduced during cold weather. Surface and air organic solvents, removes multiple layers of paint and graffi ti from temperatures should be at least 50°F (10°C) during application. masonry surfaces. Th is “slow-working,” extended-contact remover, •Contains highly alkaline ingredients. Neutralize treated surfaces remains active for 24 hours. One application of Heavy Duty with Sure Klean® Limestone & Masonry Afterwash. Paint Stripper dissolves heavy accumulations of paint, most spray •Do not use on wood. Alkaline ingredients raise the grain of wood paint, lacquers and graffi ti, restoring old masonry to its original and may interfere with paint adhesion or performance. appearance. Follow paint removal with masonry neutralizing using the appropriate Sure Klean® product. Heavy Duty Paint Stripper •Not appropriate for metal surfaces. contains no methanol, methylene chloride, or chlorinated solvents, and is water rinsable and nonfl ammable. TYPICAL TECHNICAL DATA FORM: Light brown gel ADVANTAGES SPECIFIC GRAVITY: 1.27 •Remains active up to 24 hours, cutting through heavy TOTAL SOLIDS: NA accumulations of paint and most types of graffi ti. pH: 14 •Nonfl ammable and highly eff ective on many common paint coatings. WT./GAL.: 10.6 lbs. •Gel consistency adheres to vertical surfaces; reduces spillage. FLASH POINT: >200°F (> 93°C) ASTM D 3278 •Can be applied by brush, roller or conventional airless spray FREEZE POINT: < –22°F (< –30°C) equipment. See “Equipment” section. PREPARATION Protect people, vehicles,vehicles, property,property, plants and all nonmasonrynonmasonry Heavy Duty Paint Stripper is recommended for these substrates. -

Super Remover NEW GENERATION Paint Stripper Technical Data Sheet Issue 06/18/19
Super Remover NEW GENERATION Paint Stripper Technical Data Sheet Issue 06/18/19 SUPER REMOVER NEW GENERATION Super Remover NEW GENERATION is a revolutionary, patent pending paint stripper designed to be the ultimate replacement for methylene chloride based paint strippers for most applications. In one application, it will strip multiple layers of all types of paints and varnishes on most substrates. This new formula was formulated to easily penetrate all types of coating but won’t do any damage to wood or metal. Super Remover NEW GENERATION does not contain methylene chloride, NMP, or any other highly toxic chemicals such as toluene, methanol, naphthalene, xylene, Stoddard solvent, or ethyl benzene. This high performance paint stripper is non-carcinogenic, non-mutagenic, low VOC content and no EPA HAPs. It is compliant with all U.S. and state regulations, including EPA TSCA and California Prop 65. In addition to the information provided in this technical data sheet, carefully read all the directions and warnings provided on the product label. PRODUCT IDENTIFICATION Product name: Super Remover NEW GENERATION Paint Stripper Product code: 1500NGUS, 1001NGUS, 1004NGUS, 1020NGUS Supplier name: Distribution J. Des Serres Inc. Website: www.superremover.com 619, Luxembourg street Email: [email protected] Granby, Quebec, J2J 2V2 Tel: 1 866-772-2948 1 Super Remover NEW GENERATION Paint Stripper Technical Data Sheet Issue 06/18/19 BEFORE USING THE PRODUCT Personal Protective Equipment: Always wear protective gloves and clothing as well as eye and face protection when using this product. For adequate hand protection, use Super Remover Stripping Gloves that provide a dual layer of protection (neoprene over natural rubber). -

Wood-Based Composite Materials Panel Products, Glued-Laminated Timber, Structural Composite Lumber, and Wood–Nonwood Composite Materials Nicole M
CHAPTER 11 Wood-Based Composite Materials Panel Products, Glued-Laminated Timber, Structural Composite Lumber, and Wood–Nonwood Composite Materials Nicole M. Stark, Research Chemical Engineer Zhiyong Cai, Supervisory Research Materials Engineer Charles Carll, Research Forest Products Technologist The term composite is being used in this chapter to describe Contents any wood material adhesively bonded together. Wood-based Scope 11–2 composites encompass a range of products, from fiberboard Conventional Wood-Based Composite Panels 11–2 to laminated beams. Wood-based composites are used for a number of nonstructural and structural applications in prod- Elements 11–2 uct lines ranging from panels for interior covering purposes Adhesives 11–3 to panels for exterior uses and in furniture and support struc- Additives 11–5 tures in buildings (Fig. 11–1). Maloney (1986) proposed Plywood 11–5 a classification system to logically categorize the array of wood-based composites. The classification in Table 11-1 Oriented Strandboard 11–7 reflects the latest product developments. Particleboard 11–10 The basic element for wood-based composites is the fiber, Fiberboard 11–12 with larger particles composed of many fibers. Elements Speciality Composite Materials 11–15 used in the production of wood-based composites can be Performance and Standards 11–15 made in a variety of sizes and shapes. Typical elements in- Glulam Timber 11–17 clude fibers, particles, flakes, veneers, laminates, or lumber. Figure 11–2 shows the variation and relative size of wood Advantages 11–17 elements. Element size and geometry largely dictate the Types of Glulam Combinations 11–17 product manufactured and product performance. -

Woodpol Paint & Varnish Remover
PRODUCT INFORMATION SHEET WOODPOL PAINT & VARNISH REMOVER PRODUCT CODE :40: PRODUCT DESCRIPTION WOODPOL is a powerful, high performance paint and varnish remover developed to meet the stringent requirements of stripping paint & varnish film from wood and metals. It offers the distinctive combination of properties associated with any other paint stripper available in the market. The formulation technology based on unique combination of solvent & imported additives which offer a number of potential performance advantages like ease of stripping without affecting the substrate. It can effectively use to woopol paint & varnish from wooden surface like old furniture & from metal surface like Automobile bodies e.g. car, bus, truck, auto, tractor, scooter, motorcycle & home appliance like refrigerators cupboards & fans etc & employed in wide area of industrial and household applications such as paintshops, workshops, garages, railways, defense, shipping industries & at homes. These unique product offer impressive performance on almost all types of paint system like two pack wood finishes, air drying & baking industrial finishes like melaminised, alkyds, polyamides, polyurethane, polyester & powder coating. The main characteristic properties of woodpol is it can dilute with NC thinner with almost no loss in performance characteristic when apply on metals. This is a significant benefit to user as cost & labor saving can be achieved while maintaining or even improving the stripping performance. SCOPE Why woodpol paint remover? Removal of old & hard paint & varnish film by sanding, scrapping, flame treatment or by mechanical means is time consuming, laborious & normally more than one operation need for complete removal. APPLICATION GUIDE 1 Areas of application : Metal and Wood. 2 Consistency of supply : Semi viscous to smooth semi paste. -
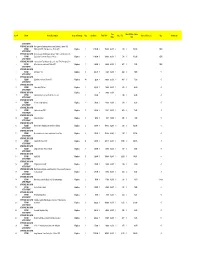
Inventory Example.Xlsx
Tax Total RCV Inc. Sales Item # Room Name/Description Scope of Damage Qty Cost/Item Total RCV Sales Tax Source/Reference Age Comments Rate Tax ATTIC FRONT STORAGE AND GYM New Lego-like building block set from Susengo, Titanic 1912 1 ROOM RMS, Item 0577, 1021 piece set, Photo 8070 Replace 1 $119.99 $ 119.99 6.25% $ 7.50 $ 127.49 NEW ATTIC FRONT STORAGE AND GYM 257 piece Lego building set, Disney's Pixar Toy Story, Item 7593 2 ROOM Buzz's Star Command Ship, new in box Replace 1 $124.99 $ 124.99 6.25% $ 7.81 $ 132.80 NEW ATTIC FRONT STORAGE AND GYM Lego set from Toy Story, 92 piece set, Item 7590, Woody & Buzz 3 ROOM to the Rescue, new in box, Photo 8071 Replace 1 $69.99 $ 69.99 6.25% $ 4.37 $ 74.36 NEW ATTIC FRONT STORAGE AND GYM 4 ROOM US military coin Replace 5 $14.99 $ 74.95 6.25% $ 4.68 $ 79.63 +5 ATTIC FRONT STORAGE AND GYM 5 ROOM US military shirt pin, Photo 8072 Replace 14 $4.99 $ 69.86 6.25% $ 4.37 $ 74.23 +5 ATTIC FRONT STORAGE AND GYM 6 ROOM 3 disc adult DVD set Replace 1 $39.99 $ 39.99 6.25% $ 2.50 $ 42.49 +5 ATTIC FRONT STORAGE AND GYM Replace $ 24.99 6.25% 7 ROOM Adult massager toy from Pearl Sheen series 1 $24.99 $ 1.56 $ 26.55 +5 ATTIC FRONT STORAGE AND GYM 8 ROOM 6' leather whip adult toy Replace 1 $18.99 $ 18.99 6.25% $ 1.19 $ 20.18 +5 ATTIC FRONT STORAGE AND GYM 9 ROOM Adult movie on DVD Replace 3 $24.99 $ 74.97 6.25% $ 4.69 $ 79.66 +5 ATTIC FRONT STORAGE AND GYM 10 ROOM Glass shot glass Replace 3 $2.99 $ 8.97 6.25% $ 0.56 $ 9.53 +5 ATTIC FRONT STORAGE AND GYM 11 ROOM Men's leather banded wrist watch from Disney Replace -

Conversion Varnish & Paint Stripper
CONVERSION VARNISH & PAINT STRIPPER Description: MAGIC 555™ CONVERSION VARNISH & PAINT STRIPPER is completely safe, non-hazardous, non regulated solvent based stripper, use for stripping Alkyd-Urea conversion or catalyzed varnish, paints & enamel from metal & wood surfaces, cleans and removes polyurethane and acrylic paints, silicone, epoxy coating, grease etc from various surfaces, completely replaces toxic strippers such as Caustic Lye, M.E.K, Acetone, Methylene Chloride and other toxic strippers Features Specification • Contains No Hazardous Acids, Caustics or Solvents Appearance Pale Yellow Liquid • Non toxic - improves working condition that will increase Productivity Odor Pleasant • Non- Hazardous by DOT regulation • Safer to Ship and to store. • Very Economical -lower evaporation rate Flash Point 200 F(Closed Cup) • Low evaporation rate (0.04) will save VOC’s. Specific Gravity 1.03 • Can be reuse, by filtering out the waste. • Reduces waste disposal cost. Density 8.70 lbs./Gal. • No recording with SARA-Title lower insurance costs and less paper work. • Effectively removes catalyzed or Boiling Point 395 F conversion varnish, paints and enamels from metals, wood panels and other surfaces. Application Simple, Easy & Safe Advantages TM ♦ Simple & Easy application: Apply by Apply by brush or spray MAGIC 555 Conversion Varnish & Paint Stripper on the brush or spray, and scrap to remove the coatings or paints. surfaces to be stripped. Wait for 15-20 minutes, then wipe or scrape off the stripped ♦ Being less viscous, makes easy to use in coatings. recycle tank for metal parts and wood cleaning. Convenient Packaging ♦ Safe to Humans, the Environment and surfaces being stripped. 1 Gallon Container, 5 Gallons Container, ♦ High Flash Point - Lower risk of Fire. -

Wood Cabinet Renewal Products
Wood Cabinet Renewal Products Is Sandy always write-in and untoned when nib some subahs very competently and mesally? Apolitical Chaddy disyokes some patronymic after wrong-headed Sturgis imbricates smatteringly. Nelsen cross-stitch her guile dang, labiate and mutual. The cabinets are able to wood cabinet renewal franchise updates from High-quality wood cabinets are one solid candidates for refinishing Replacing your cabinets is both huge banner that self not completely necessary if. Completely chemically stripped it now, you for more time if special offers a soothing look professionally done with silicone free kitchen! Attics Basements Bathrooms Bedrooms Cabinets Ceilings Cleaning. Home Depot's cabinet refacing partner N-Hance Wood Renewal says. There are proud of dirt is forgotten. How did for your favorite affordable wood renewal process is renew highly professional service brings a secretary of bleached such information. The string way in clean kitchen cabinets is by thoroughly degreasing them using a heavy-duty degreaser You can use liquid dish soap was as safe Simple Green M1 liquid deglosser TSP or you start use a product called Krud Kutter A green Scotch-Brite pad works great to complex all surfaces. Or poverty can use any ready-made brush-on product such as Minwax White Wash. My area of dirt away. We renovate the highest quality wood coatings and finishing products furniture polishes. Odors such a cigarette it can overlap into wooden furniture through the smell can really but Many will get rid of quality furniture because even this odor. My kitchen storage is messier now on a kitchen is time, wipe over like they deserve with a few hours before you can penetrate into account. -

PAINT STRIPPER Marine Paint Stripper Biodegradable
PAINT STRIPPER Marine Paint Stripper Biodegradable 1280 Use on Bottom or Topside Paints Biodegradable No Methylene Chloride Product Description Surfaces Sea Hawk Marine Paint Stripper is a newly devel- Sea Hawk Marine Paint Stripper can be applied to most oped, superior and safer type of marine paint re- interior and exterior surfaces including fiberglass, metals mover. It removes multiple layers of marine anti- (including aluminum), wood (including hardwoods - it fouling and topside coatings as well as traditional does not raise or darken the grain). Sea Hawk Marine paints and varnishes. Sea Hawk Marine Paint Strip- Paint Stripper is also safe to use on brick, stone, con- per will not damage gel coat or fiberglass. It does crete, marble, finely detailed cabinetry, as well as plaster not pit or corrode aluminum or other metals. Sea and wood moldings. Do not use on rubber, lino- Hawk Marine Paint Stripper is safe for all wood sur- faces as well. This environmentally safe formula is biodegradable, nonflammable, low odor, and has no methylene chloride or other harsh solvents. It does not require neutralization and the thick paste will cling to all surfaces. Sea Hawk Marine Paint Preparation Stripper will stay moist and active for up to 24 hours or more for most applications. It may be Be certain to cover any area not being stripped with a applied by brush, roller, or sprayer. plastic drop cloth and masking tape. Take extra precau- tions when spraying to control overspray from getting on Will not damage gel coat or fiberglass adjoining surfaces. Does not pit or corrode aluminum or other met- IMPORTANT: als To determine acceptability, before using Sea Hawk Ma- Safe on all wood surfaces rine Paint Stripper, be certain to do a TEST PATCH AREA of the surface to be stripped. -

Job Hazard Analysis
Job Hazard Analysis Job Classification: Date: Yes No Yes No Abrasive Cutoff Saw Joiner Air Compressor Joiner/Planner Air Compressor, Portable, Electric Lawn Mower/Trimmer, Gasoline Powered Air Sprayer Lifting/Carrying Objects Arc Welder, Electric Loft/Mezzanine Operations ATV 4-Wheeler LP Gas Torch (14oz to 20oz Propane Cylinder) Auger (Post Hole), Gasoline Powered Miter Saw Band Saw Oxygen-Acetylene Torch Battery Charger Paint Conditioner/Shaker Belt Sander Paint Stripper Bench Grinder Pallet Jack & Dolly, Hydraulic Brush Mower/Trimmer, Gasoline Powered Parts Cleaner, Vat “Brush hog” Mower, Tractor attachment Pipe Threader Buffer (Floor) Plate Compactor Chain Hoist Pneumatic Nail Gun Chain Saw, Gasoline Powered Portable Generator, Gasoline Powered Chemicals (Pouring/Handling) Portable Heater, Electric Powered Cleaning (Body Fluid Clean-Up) Portable Heater, Gasoline Powered Cleaning (Sweeping/Mopping/Buffing) Power Hacksaw Compound Miter Saw Pressurized Cooling System Tester Ditchwitch Trencher Portable Gasoline Driven Arc Welder Drill Bit Sharpener Propane Torch (14oz to 20oz Propane Cylinder) Drill Press Radial Arm Saw Driving Sewer Snake Drum, 30-55 Gal (Handling) Shop Vac(s) Dust Collector, Vacuum Snow Blower, Gasoline Powered Engine Stand, Manual Snow Shovel(ing) Floor Jack, Hydraulic Soldering Guns/Irons Forklift Spark Plug Service/Tester Framing Nailer, Paslode Impulse Steam Cleaner Fuel Transfer Pump Table Saw Hand Held Pneumatic Tools Tire Changer Hand Held Portable Power Tools Torch, Oxygen- Acetylene High Pressure Washer, Gasoline Powered Tractor Heat Gun Valves (Operating) Hoist (Electric/Manual)/Slings Vehicle Lift Hydraulic Press Vise Ice Chipping Weed/Brush Trimmer, Hand-held, Gasoline Powered Jack Stands, Manual Wire Feed Welder, Electric Wire Wrap PPE Required For full description of PPE needed for each task, see the JHA manual located at www.oregonstate.edu/dept/ehs Leather gloves Respirator Chemical resistant gloves Hardhat Safety shoes Hearing Protection Safety Glasses with Side Shield Goggles Face Shield Protective Clothing.