FOI 215-1718 Document 5
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(12) Patent Application Publication (10) Pub. No.: US 2006/0110428A1 De Juan Et Al
US 200601 10428A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0110428A1 de Juan et al. (43) Pub. Date: May 25, 2006 (54) METHODS AND DEVICES FOR THE Publication Classification TREATMENT OF OCULAR CONDITIONS (51) Int. Cl. (76) Inventors: Eugene de Juan, LaCanada, CA (US); A6F 2/00 (2006.01) Signe E. Varner, Los Angeles, CA (52) U.S. Cl. .............................................................. 424/427 (US); Laurie R. Lawin, New Brighton, MN (US) (57) ABSTRACT Correspondence Address: Featured is a method for instilling one or more bioactive SCOTT PRIBNOW agents into ocular tissue within an eye of a patient for the Kagan Binder, PLLC treatment of an ocular condition, the method comprising Suite 200 concurrently using at least two of the following bioactive 221 Main Street North agent delivery methods (A)-(C): Stillwater, MN 55082 (US) (A) implanting a Sustained release delivery device com (21) Appl. No.: 11/175,850 prising one or more bioactive agents in a posterior region of the eye so that it delivers the one or more (22) Filed: Jul. 5, 2005 bioactive agents into the vitreous humor of the eye; (B) instilling (e.g., injecting or implanting) one or more Related U.S. Application Data bioactive agents Subretinally; and (60) Provisional application No. 60/585,236, filed on Jul. (C) instilling (e.g., injecting or delivering by ocular ion 2, 2004. Provisional application No. 60/669,701, filed tophoresis) one or more bioactive agents into the Vit on Apr. 8, 2005. reous humor of the eye. Patent Application Publication May 25, 2006 Sheet 1 of 22 US 2006/0110428A1 R 2 2 C.6 Fig. -

The Effects of Antipsychotic Treatment on Metabolic Function: a Systematic Review and Network Meta-Analysis
The effects of antipsychotic treatment on metabolic function: a systematic review and network meta-analysis Toby Pillinger, Robert McCutcheon, Luke Vano, Katherine Beck, Guy Hindley, Atheeshaan Arumuham, Yuya Mizuno, Sridhar Natesan, Orestis Efthimiou, Andrea Cipriani, Oliver Howes ****PROTOCOL**** Review questions 1. What is the magnitude of metabolic dysregulation (defined as alterations in fasting glucose, total cholesterol, low density lipoprotein (LDL) cholesterol, high density lipoprotein (HDL) cholesterol, and triglyceride levels) and alterations in body weight and body mass index associated with short-term (‘acute’) antipsychotic treatment in individuals with schizophrenia? 2. Does baseline physiology (e.g. body weight) and demographics (e.g. age) of patients predict magnitude of antipsychotic-associated metabolic dysregulation? 3. Are alterations in metabolic parameters over time associated with alterations in degree of psychopathology? 1 Searches We plan to search EMBASE, PsycINFO, and MEDLINE from inception using the following terms: 1 (Acepromazine or Acetophenazine or Amisulpride or Aripiprazole or Asenapine or Benperidol or Blonanserin or Bromperidol or Butaperazine or Carpipramine or Chlorproethazine or Chlorpromazine or Chlorprothixene or Clocapramine or Clopenthixol or Clopentixol or Clothiapine or Clotiapine or Clozapine or Cyamemazine or Cyamepromazine or Dixyrazine or Droperidol or Fluanisone or Flupehenazine or Flupenthixol or Flupentixol or Fluphenazine or Fluspirilen or Fluspirilene or Haloperidol or Iloperidone -

Antipsychotics and the Risk of Sudden Cardiac Death
ORIGINAL INVESTIGATION Antipsychotics and the Risk of Sudden Cardiac Death Sabine M. J. M. Straus, MD; Gyse`le S. Bleumink, MD; Jeanne P. Dieleman, PhD; Johan van der Lei, MD, PhD; Geert W. ‘t Jong, PhD; J. Herre Kingma, MD, PhD; Miriam C. J. M. Sturkenboom, PhD; Bruno H. C. Stricker, PhD Background: Antipsychotics have been associated with Results: The study population comprised 554 cases of prolongation of the corrected QT interval and sudden car- sudden cardiac death. Current use of antipsychotics was diac death. Only a few epidemiological studies have in- associated with a 3-fold increase in risk of sudden car- vestigated this association. We performed a case- diac death. The risk of sudden cardiac death was high- control study to investigate the association between use est among those using butyrophenone antipsychotics, of antipsychotics and sudden cardiac death in a well- those with a defined daily dose equivalent of more than defined community-dwelling population. 0.5 and short-term (Յ90 days) users. The association with current antipsychotic use was higher for witnessed cases Methods: We performed a population-based case-control (n=334) than for unwitnessed cases. study in the Integrated Primary Care Information (IPCI) project, a longitudinal observational database with com- Conclusions: Current use of antipsychotics in a gen- plete medical records from 150 general practitioners. All eral population is associated with an increased risk of sud- instances of death between January 1, 1995, and April 1, den cardiac death, even at a low dose and for indica- 2001, were reviewed. Sudden cardiac death was classified tions other than schizophrenia. -

Importance of the Newer Generations Of
Psychiatria Danubina, 2013; Vol. 25, No. 3, pp 329-333 Conference paper © Medicinska naklada - Zagreb, Croatia IMPORTANCE OF THE NEWER GENERATIONS OF ANTIPSYCHOTICS IN REDUCING SCHIZOPHRENIA HOSPITALIZATION RATES Vlado Jukić, Aleksandar Savić & Miroslav Herceg University Psychiatric Hospital Vrapče, Zagreb, Croatia SUMMARY Throughout history, given the lack of understanding of schizophrenia and lack of effective treatment options, patients were often committed to asylums and later psychiatric institutions, often for prolonged periods of time. First antipsychotic medications helped to bring about changes in approach to these patients and facilitated deinstitutionalization, and discovery of new drugs with differing side-effects profiles introduced new options in treating schizophrenia patients. Data on hospitalization of patients in University Psychiatric Hospital Vrapče from the mid-1990s, as well as data on national level, suggests a trend of drop in hospitalization of schizophrenia patients. At the same time, that period saw significant increase in a number of available newer-generations antipsychotics and the rise in their use compared to first-generation one. Although far from being the only contributing factor, seem to play an important role in continuing the trend of reducing hospitalization rates for schizophrenia patients that started with first antipsychotics. Newer antipsychotics with a more tolerable side-effects profile promote better compliance and further reduce rate of relapse and hospitalizations. No less important is the contribution of newer antipsychotics with new receptor profiles to the personalized psychopharmacotherapy approach that is in tune with emerging conceptualizations of schizophrenia as a complex syndrome with a number of separate symptom domains, whose specific combinations produce specific individual clinical presentation and in turn ask for a specific individual approach to the patient. -

Drug and Medication Classification Schedule
KENTUCKY HORSE RACING COMMISSION UNIFORM DRUG, MEDICATION, AND SUBSTANCE CLASSIFICATION SCHEDULE KHRC 8-020-1 (11/2018) Class A drugs, medications, and substances are those (1) that have the highest potential to influence performance in the equine athlete, regardless of their approval by the United States Food and Drug Administration, or (2) that lack approval by the United States Food and Drug Administration but have pharmacologic effects similar to certain Class B drugs, medications, or substances that are approved by the United States Food and Drug Administration. Acecarbromal Bolasterone Cimaterol Divalproex Fluanisone Acetophenazine Boldione Citalopram Dixyrazine Fludiazepam Adinazolam Brimondine Cllibucaine Donepezil Flunitrazepam Alcuronium Bromazepam Clobazam Dopamine Fluopromazine Alfentanil Bromfenac Clocapramine Doxacurium Fluoresone Almotriptan Bromisovalum Clomethiazole Doxapram Fluoxetine Alphaprodine Bromocriptine Clomipramine Doxazosin Flupenthixol Alpidem Bromperidol Clonazepam Doxefazepam Flupirtine Alprazolam Brotizolam Clorazepate Doxepin Flurazepam Alprenolol Bufexamac Clormecaine Droperidol Fluspirilene Althesin Bupivacaine Clostebol Duloxetine Flutoprazepam Aminorex Buprenorphine Clothiapine Eletriptan Fluvoxamine Amisulpride Buspirone Clotiazepam Enalapril Formebolone Amitriptyline Bupropion Cloxazolam Enciprazine Fosinopril Amobarbital Butabartital Clozapine Endorphins Furzabol Amoxapine Butacaine Cobratoxin Enkephalins Galantamine Amperozide Butalbital Cocaine Ephedrine Gallamine Amphetamine Butanilicaine Codeine -

Marrakesh Agreement Establishing the World Trade Organization
No. 31874 Multilateral Marrakesh Agreement establishing the World Trade Organ ization (with final act, annexes and protocol). Concluded at Marrakesh on 15 April 1994 Authentic texts: English, French and Spanish. Registered by the Director-General of the World Trade Organization, acting on behalf of the Parties, on 1 June 1995. Multilat ral Accord de Marrakech instituant l©Organisation mondiale du commerce (avec acte final, annexes et protocole). Conclu Marrakech le 15 avril 1994 Textes authentiques : anglais, français et espagnol. Enregistré par le Directeur général de l'Organisation mondiale du com merce, agissant au nom des Parties, le 1er juin 1995. Vol. 1867, 1-31874 4_________United Nations — Treaty Series • Nations Unies — Recueil des Traités 1995 Table of contents Table des matières Indice [Volume 1867] FINAL ACT EMBODYING THE RESULTS OF THE URUGUAY ROUND OF MULTILATERAL TRADE NEGOTIATIONS ACTE FINAL REPRENANT LES RESULTATS DES NEGOCIATIONS COMMERCIALES MULTILATERALES DU CYCLE D©URUGUAY ACTA FINAL EN QUE SE INCORPOR N LOS RESULTADOS DE LA RONDA URUGUAY DE NEGOCIACIONES COMERCIALES MULTILATERALES SIGNATURES - SIGNATURES - FIRMAS MINISTERIAL DECISIONS, DECLARATIONS AND UNDERSTANDING DECISIONS, DECLARATIONS ET MEMORANDUM D©ACCORD MINISTERIELS DECISIONES, DECLARACIONES Y ENTEND MIENTO MINISTERIALES MARRAKESH AGREEMENT ESTABLISHING THE WORLD TRADE ORGANIZATION ACCORD DE MARRAKECH INSTITUANT L©ORGANISATION MONDIALE DU COMMERCE ACUERDO DE MARRAKECH POR EL QUE SE ESTABLECE LA ORGANIZACI N MUND1AL DEL COMERCIO ANNEX 1 ANNEXE 1 ANEXO 1 ANNEX -

Preparation of Metabolites by Chemical Reaction: Conversion of Antipsychotic Phenothiazines to Their Sulfoxides and Tertiary
396 Journal of Health Science, 50(4) 396–406 (2004) Preparation of Metabolites N-oxides from parent drug could be confirmed when the collisional energy was decreased to 10 eV. 1H- and by Chemical Reaction: 13C-NMR spectral data confirmed the structure of the Conversion of Antipsychotic prepared mianserin N-oxide to be 2-oxide. In conclu- sion, a simple and rapid preparation method for oxide Phenothiazines to their metabolites of phenothiazines and tertiary amino cy- Sulfoxides and Tertiary clic antidepressants available as analytical standards was established. Amino Cyclic Key words —–— metabolite, chemical preparation, ox- Antidepressants ide, phenothiazine, cyclic antidepressants, mass spectros- to their N-Oxide with copy Hydrogen Peroxide Using Titanosilicate INTRODUCTION Catalyst The identification and quantification of drugs Akira Ogamo* and Mariko Fukumoto ingested into human bodies require comprehensive information covering the target drugs themselves and School of Pharmaceutical Sciences, Kitasato University, their metabolites. To carry out a quantitative analy- Shirokane 5–9–1, Minato-ku, Tokyo 108–8641, Japan sis, the standard substances of metabolites are es- (Received March 5, 2004; Accepted April 11, 2004) sential. Some important metabolites can be obtained as commercial products, but many metabolites, es- The aim of this study was to establish a prepara- pecially those of new drugs, are difficult to obtain tion method needed to analyze the metabolites of an as standard samples. These problems have been analytical procedure for antipsychotic phenothiazines solved by using experimental animals or isolated and tertiary amino cyclic antidepressants by chemical enzyme systems originating from biological mate- reaction. These drugs were oxidized to their sulfoxide rials. -

Gianluca BW.Indd
Use and safety of psychotropic drugs in elderly patients Gianluca Trifi rò GGianlucaianluca BBW.inddW.indd 1 225-May-095-May-09 111:22:461:22:46 AAMM Th e work presented in this thesis was conducted both at the Department of Medical Informatics of the Erasmus Medical Center, Rotterdam, Th e Netherlands and the Depart- ment of Clinical and Experimental Medicine and Pharmacology of University of Messina, Messina, Italy. Th e studies reported in chapter 2.2 and 4.3 were respectively supported by grants from the Italian Drug Agency and Pfi zer. Th e contributions of the general practitioners participating in the IPCI, Health Search/ Th ales, and Arianna databases are greatly acknowledged. Financial support for printing and distribution of this thesis was kindly provided by IPCI, SIMG/Health Search, Eli Lilly Nederland BV, Boehringer-Ingelheim B.V., Novartis Pharma B.V. and University of Messina. Cover design by Emanuela Punzo, Antonio Franco e Gianluca Trifi rò. Th e background photo in the cover is a landscape from Santa Lucia del Mela (Sicily) and was kindly pro- vided by Franco Trifi rò. Th e photo of the elderly person is from Antonio Franco Trifi rò. Layout and print by Optima Grafi sche Communicatie, Rotterdam, Th e Netherlands. GGianlucaianluca BBW.inddW.indd 2 225-May-095-May-09 111:22:481:22:48 AAMM Use and Safety of Psychotropic Drugs in Elderly Patients Gebruik en veiligheid van psychotropische medicaties in de oudere patienten Proefschrift ter verkrijging van de graad van doctor aan de Erasmus Universiteit Rotterdam op gezag van de rector magnifi cus Prof.dr. -
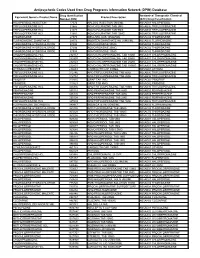
Antipsychotic Codes Used from Drug Programs Information Network (DPIN) Database
Antipsychotic Codes Used from Drug Programs Information Network (DPIN) Database Drug Identification Anatomical Therapeutic Chemical Equivalent Generic Product Name Product Description Number (DIN) (ATC) Drug Classification HALOPERIDOL INJECTION 17574 HALDOL INJECTION 5MG/ML N05AD01 HALOPERIDOL TRIFLUOPERAZINE HCL 21865 NOVO-FLURAZINE TAB 2MG N05AB06 TRIFLUOPERAZINE TRIFLUOPERAZINE HCL 21873 NOVO-FLURAZINE TAB 5MG N05AB06 TRIFLUOPERAZINE TRIFLUOPERAZINE HCL 21881 NOVO-FLURAZINE TAB 10MG N05AB06 TRIFLUOPERAZINE THIORIDAZINE 27375 MELLARIL SUS 10MG/5ML N05AC02 THIORIDAZINE FLUPHENAZINE ENANTHATE 29173 MODITEN ENANTHATE INJ 25MG/ML N05AB02 FLUPHENAZINE THIORIDAZINE HYDROCHLORIDE 37486 NOVO-RIDAZINE 50MG N05AC02 THIORIDAZINE THIORIDAZINE HYDROCHLORIDE 37494 NOVO-RIDAZINE 25MG N05AC02 THIORIDAZINE THIORIDAZINE HYDROCHLORIDE 37508 NOVO-RIDAZINE 10MG N05AC02 THIORIDAZINE CHLORPROMAZINE HCL 232157 NOVO-CHLORPROMAZINE TAB 10MG N05AA01 CHLORPROMAZINE CHLORPROMAZINE HCL 232807 NOVO-CHLORPROMAZINE TAB 50MG N05AA01 CHLORPROMAZINE CHLORPROMAZINE HCL 232823 NOVO-CHLORPROMAZINE TAB 25MG N05AA01 CHLORPROMAZINE CHLORPROMAZINE HCL 232831 NOVO-CHLORPROMAZINE TAB 100MG N05AA01 CHLORPROMAZINE LITHIUM CARBONATE 236683 CARBOLITH CAP 300MG N05AN01 LITHIUM TRIFLUOPERAZINE HCL 312746 APO TRIFLUOPERAZINE TAB 5MG N05AB06 TRIFLUOPERAZINE TRIFLUOPERAZINE HCL 312754 APO TRIFLUOPERAZINE TAB 2MG N05AB06 TRIFLUOPERAZINE PIMOZIDE 313815 ORAP TAB 2MG N05AG02 PIMOZIDE PIMOZIDE 313823 ORAP TAB 4MG N05AG02 PIMOZIDE TRIFLUOPERAZINE HCL 326836 APO TRIFLUOPERAZINE TAB 10MG N05AB06 -

Drugs Which Can Affect Near Vision: a Useful List
Drugs Which Can Affect Near Vision: A Useful List Joanne L. Smith B.Sc., Ph.Phm.* J. Raymond Buncic, M.D., F.R.C.S.(C)t ABSTRACT This paper documents a list of drugs that cause problems with near vision, by virtue of effects on accommodation, occasionally refractive error and diplopia. It is meant as a reference aid to the clinician when confronted with problems of focusing on near objects or print. There are many drugs that have been reported to interfere with near or reading vision, producing blurring, decreased accommodation and diplopia. This paper lists the drugs that have been reported in the literature to produce symptoms which interfere with near vision. Case reports for the listed drugs vary greatly from many to few. The drugs have been divided into the following categories: those causing (A) blurring at near, (B) diplopia and (C) induced myopia. Those drugs which only rarely cause these symptoms have been omitted. From the Departments of Pharmacy* and Ophthalmologyt, The Hospital For Sick Children, Toronto, Ontario, Canada Requests for reprints should be addressed to: Dr. J. Raymond Buncic, Department of Ophthalmology, The Hospital For Sick Children, 555 University Ave., Toronto, Ontario, Canada M5G lX8 TABLE 1 DRUGS COMMONLY CAUSING DIFFICULTY WITH FOCUSING AT NEAR OR BLURRED VISION. DRUG INCIDENCE REFERENCE Antipsychotics Chlorpromazine 14-23 8 Clozapine 5 8,14 Fluphenazine 1.2-4.3 8 Haloperidol 6.8-16 8 Loxapine 12,14 Perphenazine 7.4-17.8 8 Pimozide 20 8 Risperidone 1-2%, >/= 10% 11 Thioridazine 0.6-18 8 Thiothixene 20 8 -

Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DIX to the HTSUS—Continued
20558 Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DEPARMENT OF THE TREASURY Services, U.S. Customs Service, 1301 TABLE 1.ÐPHARMACEUTICAL APPEN- Constitution Avenue NW, Washington, DIX TO THE HTSUSÐContinued Customs Service D.C. 20229 at (202) 927±1060. CAS No. Pharmaceutical [T.D. 95±33] Dated: April 14, 1995. 52±78±8 ..................... NORETHANDROLONE. A. W. Tennant, 52±86±8 ..................... HALOPERIDOL. Pharmaceutical Tables 1 and 3 of the Director, Office of Laboratories and Scientific 52±88±0 ..................... ATROPINE METHONITRATE. HTSUS 52±90±4 ..................... CYSTEINE. Services. 53±03±2 ..................... PREDNISONE. 53±06±5 ..................... CORTISONE. AGENCY: Customs Service, Department TABLE 1.ÐPHARMACEUTICAL 53±10±1 ..................... HYDROXYDIONE SODIUM SUCCI- of the Treasury. NATE. APPENDIX TO THE HTSUS 53±16±7 ..................... ESTRONE. ACTION: Listing of the products found in 53±18±9 ..................... BIETASERPINE. Table 1 and Table 3 of the CAS No. Pharmaceutical 53±19±0 ..................... MITOTANE. 53±31±6 ..................... MEDIBAZINE. Pharmaceutical Appendix to the N/A ............................. ACTAGARDIN. 53±33±8 ..................... PARAMETHASONE. Harmonized Tariff Schedule of the N/A ............................. ARDACIN. 53±34±9 ..................... FLUPREDNISOLONE. N/A ............................. BICIROMAB. 53±39±4 ..................... OXANDROLONE. United States of America in Chemical N/A ............................. CELUCLORAL. 53±43±0 -

DOMASYS Standard
New Zealand Data Sheet 04 December 2018 Neulactil-periciazine DATA SHEET 1 NEULACTIL TABLETS Neulactil 2.5 mg tablets Neulactil 10 mg tablets 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Periciazine 2.5mg Periciazine 10mg Excipients with known effect: lactose monohydrate and wheat starch For the full list of excipients, see section 6.1. 3 PHARMACEUTICAL FORM Neulactil tablets 2.5 mg: yellow, scored, marked NEULACTIL 10 mg: yellow, scored, marked 10 4 CLINICAL PARTICULARS 4.1 THERAPEUTIC INDICATIONS Periciazine is indicated: 1. In adults with schizophrenia or other psychoses, for the treatment of symptoms or prevention of relapse. 2. In anxiety, psychomotor agitation, violent or dangerously impulsive behaviour. periciazine is used as an adjunct to the short-term management of these conditions. Periciazine tablets are not recommended for children (see Section 4.3 and 4.4). neulactil-ccsiv4-dsv11-04dec18 Page 1 New Zealand Data Sheet 04 December 2018 Neulactil-periciazine 4.2 DOSE AND METHOD OF ADMINISTRATION Dosage requirements vary with the individual and the severity of the condition being treated. Initial dosage should be low with progressive increases until the desired response is obtained, after which dosage should be adjusted to maintain control of symptoms. Severe conditions (Indication 1) Adults Initially 75 mg per day in divided doses. Dosage should be increased by 25 mg per day at weekly intervals until optimum effect is achieved. Maintenance therapy would not normally be expected to exceed 300mg per day. Elderly Initially 15-30 mg per day in divided doses. If this is well tolerated the dosage may be increased if necessary for optimum control of behaviour.