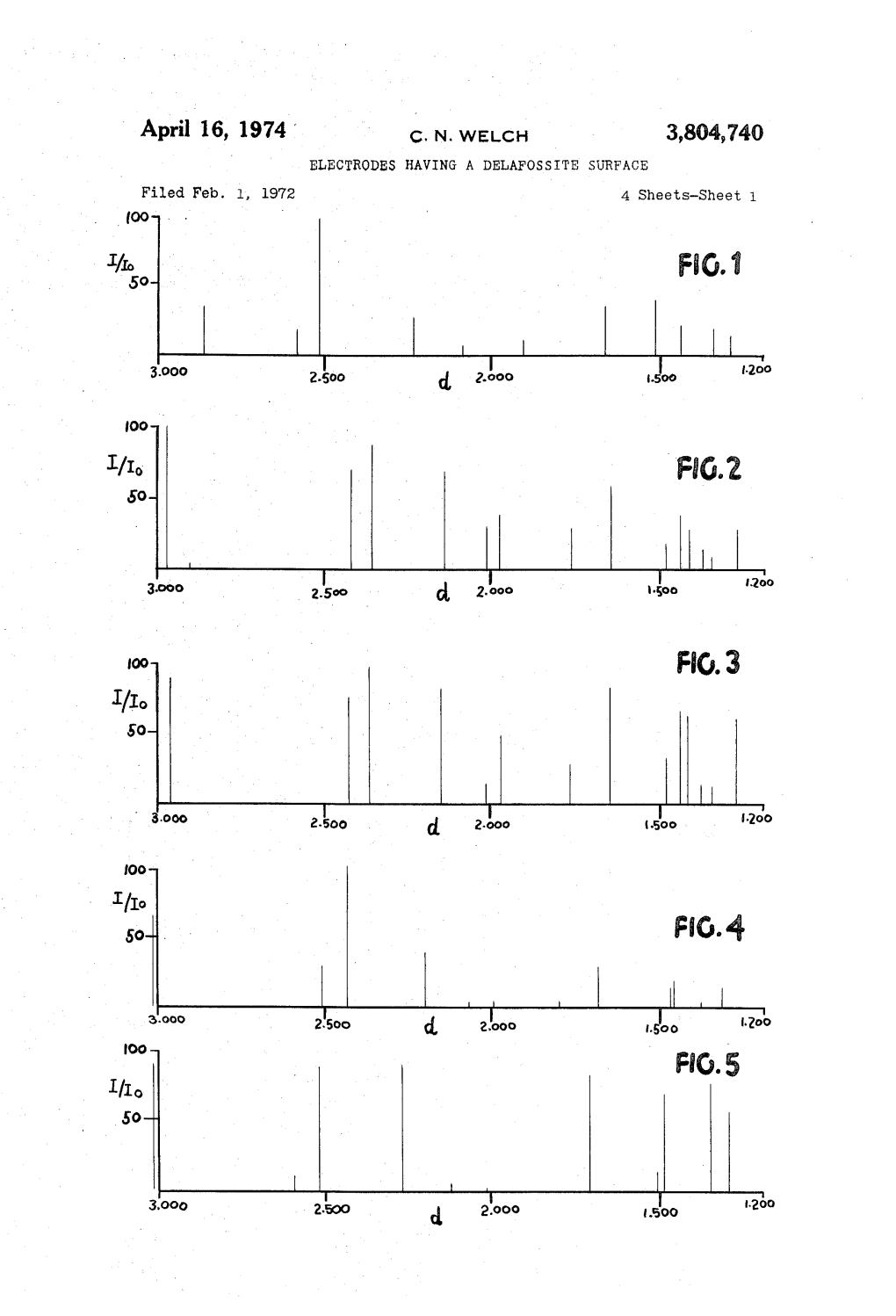
April 16, 1974 C. N. WELCH 3,804,740 Ao
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thermoelectric and Transport Properties of Delafossite Cucro2
Thermoelectric and transport properties of Delafossite CuCrO2:Mg thin films prepared by RF magnetron sputtering Inthuga Sinnarasa, Yohann Thimont, Lionel Presmanes, Antoine Barnabé, Philippe Tailhades To cite this version: Inthuga Sinnarasa, Yohann Thimont, Lionel Presmanes, Antoine Barnabé, Philippe Tailhades. Ther- moelectric and transport properties of Delafossite CuCrO2:Mg thin films prepared by RF magnetron sputtering. Nanomaterials, MDPI, 2017, 7 (7), pp.157. 10.3390/nano7070157. hal-01754780 HAL Id: hal-01754780 https://hal.archives-ouvertes.fr/hal-01754780 Submitted on 30 Mar 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Open Archive TOULOUSE Archive Ouverte ( OATAO ) OATAO is an open access repository that collects the work of Toulouse researchers and makes it freely available over the web where possible. This is a publisher’s version published in : http://oatao.univ-toulouse.fr/ Eprints ID : 19788 To link to this article : DOI:10.3390/nano7070157 URL : http://dx.doi.org/10.3390/nano7070157 To cite this version : Sinnarasa, Inthuga and Thimont, Yohann and Presmanes, Lionel and Tailhades, Philippe Thermoelectric and transport properties of Delafossite CuCrO2:Mg thin films prepared by RF magnetron sputtering . (2017) Nanomaterials, vol. -

Delafossite Cu1+Fe3+O2
1+ 3+ Delafossite Cu Fe O2 c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Hexagonal. Point Group: 32/m. Crystals tabular on {0001}, to 8 mm; also equant, bounded by {0001} and {1011}; as botryoidal crusts, spherulitic, powdery, massive. Twinning: On {0001}, as contact twins. Physical Properties: Cleavage: {1011}, imperfect. Tenacity: Brittle. Hardness = 5.5 D(meas.) = 5.41 D(calc.) = 5.52 Weakly magnetic. Optical Properties: Opaque. Color: Black; pale brownish rose in reflected light. Streak: Black. Luster: Metallic. Optical Class: Uniaxial. Pleochroism: Distinct; O = pale golden brown; E = rose-brown. Anisotropism: Strong. R1–R2: (400) 18.4–22.2, (420) 18.6–22.3, (440) 18.8–22.4, (460) 19.0–22.6, (480) 19.0–22.9, (500) 18.9–23.2, (520) 19.0–23.4, (540) 19.0–23.4, (560) 19.1–23.2, (580) 19.2–23.0, (600) 19.3–23.0, (620) 19.6–23.2, (640) 20.0–23.5, (660) 20.6–23.8, (680) 21.1–24.2, (700) 21.8–24.8 Cell Data: Space Group: R3m. a = 3.02–3.04 c = 17.10–17.12 Z = 3 X-ray Powder Pattern: Locality unknown. (ICDD 12-752). 2.508 (100), 1.512 (40), 2.86 (35), 1.658 (35), 2.238 (25), 1.434 (20), 2.58 (18) Chemistry: (1) (2) (3) Cu 42.14 41.32 41.97 Fe 33.56 37.26 36.89 O [19.74] [21.21] 21.14 rem. 3.52 0.21 Total [98.96] [100.00] 100.00 (1) Yekaterinburg, Russia; remnant is Al2O3. -

Metal Oxide-Based Transparent Conducting Oxides Meagen Anne Gillispie Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 2006 Metal oxide-based transparent conducting oxides Meagen Anne Gillispie Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Materials Science and Engineering Commons Recommended Citation Gillispie, Meagen Anne, "Metal oxide-based transparent conducting oxides " (2006). Retrospective Theses and Dissertations. 1891. https://lib.dr.iastate.edu/rtd/1891 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. UMI Number: 3243848 UMI Microform 3243848 Copyright 2007 by ProQuest Information and Learning Company. All rights reserved. This microform edition is protected against unauthorized copying under Title 17, United States Code. ProQuest Information and Learning Company 300 North Zeeb Road P.O. Box 1346 Ann Arbor, MI 48106-1346 Metal oxide-based transparent conducting oxides by Meagen Anne Gillispie A dissertation submitted to the graduate faculty in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY Major: Materials Science and Engineering Program of Study Committee: David Cann, Co-major Professor Xiaoli Tan, Co-major Professor Mufit Akinc Vikram -

Insights on the Stability and Cationic Nonstoichiometry of Cufeo2 Delafossite
Open Archive Toulouse Archive Ouverte OATAO is an open access repository that collects the work of Toulouse researchers and makes it freely available over the web where possible This is an author’s version published in: http://oatao.univ-toulouse.fr/24270 Official URL: https://doi.org/10.1021/acs.inorgchem.9b00651 To cite this version: Pinto, Juliano Schorne and Cassayre, Laurent and Presmanes, Lionel and Barnabé, Antoine Insights on the Stability and Cationic Nonstoichiometry of CuFeO2 Delafossite. (2019) Inorganic Chemistry, 58 (9). 6431-6444. ISSN 0020-1669 Any correspondence concerning this service should be sent to the repository administrator: [email protected] Insights on the Stability and Cationic Nonstoichiometry of CuFeO2 Delafossite Juliano Schorne-Pinto,†,‡ Laurent Cassayre,† Lionel Presmanes,‡ and Antoine Barnabe*́,‡ † Laboratoire de Genié Chimique, Universitéde Toulouse, CNRS, Toulouse, France ‡ CIRIMAT, Universitéde Toulouse, CNRS, UniversitéPaul Sabatier, 118 Route de Narbonne, 31062 Toulouse, Cedex 9, France *S Supporting Information ABSTRACT: CuFeO2, the structure prototype of the delafossite family, has received renewed interest in recent years. Thermodynamic modeling and several experimental Cu−Fe−O system investigations did not focus specifically on the possible nonstoichiometry of this compound, which is, nevertheless, a very important optimization factor for its physicochemical properties. In this work, through a complete set of analytical and thermostructural techniques from 50 to 1100 °C, a fine reinvestigation of some specific regions of the Cu−Fe−O phase diagram under air was carried out to clarify discrepancies concerning the delafossite CuFeO2 stability region as well as the eutectic composition and temperature for the reaction L = ff ’ CuFeO2 +Cu2O. -

Interfacial Stabilization for Epitaxial Cucro2 Delafossites
www.nature.com/scientificreports OPEN Interfacial stabilization for epitaxial CuCrO2 delafossites Jong Mok Ok1,3, Sangmoon Yoon1,3, Andrew R. Lupini2, Panchapakesan Ganesh2, Matthew F. Chisholm2 & Ho Nyung Lee1* ABO2 delafossites are fascinating materials that exhibit a wide range of physical properties, including giant Rashba spin splitting and anomalous Hall efects, because of their characteristic layered structures composed of noble metal A and strongly correlated BO2 sublayers. However, thin flm synthesis is known to be extremely challenging owing to their low symmetry rhombohedral structures, which limit the selection of substrates for thin flm epitaxy. Hexagonal lattices, such as those provided by Al2O3(0001) and (111) oriented cubic perovskites, are promising candidates for epitaxy of delafossites. However, the formation of twin domains and impurity phases is hard to suppress, and the nucleation and growth mechanisms thereon have not been studied for the growth of epitaxial delafossites. In this study, we report the epitaxial stabilization of a new interfacial phase formed during pulsed-laser epitaxy of (0001)-oriented CuCrO2 epitaxial thin flms on Al2O3 substrates. Through a combined study using scanning transmission electron microscopy/electron- energy loss spectroscopy and density functional theory calculations, we report that the nucleation of a thermodynamically stable, atomically thick CuCr1−xAlxO2 interfacial layer is the critical element for the epitaxy of CuCrO2 delafossites on Al2O3 substrates. This fnding provides key insights into the thermodynamic mechanism for the nucleation of intermixing-induced bufer layers that can be used for the growth of other noble-metal-based delafossites, which are known to be challenging due to the difculty in initial nucleation. -

Transparent Conducting Oxides—An Up-To-Date Overview
Materials 2012, 5, 661-683; doi:10.3390/ma5040661 OPEN ACCESS materials ISSN 1996-1944 www.mdpi.com/journal/materials Article Transparent Conducting Oxides—An Up-To-Date Overview Andreas Stadler University of Salzburg, Hellbrunner Str. 34, Salzburg A-5020, Austria; E-Mail: [email protected]; Tel.: +43-662-8044-2111; Fax: +43-662-8044-622 Received: 20 January 2012; in revised form: 9 March 2012 / Accepted: 28 March 2012 / Published: 19 April 2012 Abstract: Transparent conducting oxides (TCOs) are electrical conductive materials with comparably low absorption of electromagnetic waves within the visible region of the spectrum. They are usually prepared with thin film technologies and used in opto-electrical apparatus such as solar cells, displays, opto-electrical interfaces and circuitries. Here, based on a modern database-system, aspects of up-to-date material selections and applications for transparent conducting oxides are sketched, and references for detailed information are given. As n-type TCOs are of special importance for thin film solar cell production, indium-tin oxide (ITO) and the reasonably priced aluminum-doped zinc oxide (ZnO:Al), are discussed with view on preparation, characterization and special occurrences. For completion, the recently frequently mentioned typical p-type delafossite TCOs are described as well, providing a variety of references, as a detailed discussion is not reasonable within an overview publication. Keywords: transparent conducting oxide; oxide; TCO; ITO; ZnO:Al; delafossite 1. Introduction Transparent conducting oxides (TCOs) are electrical conductive materials with a comparably low absorption of light. They are usually prepared with thin film technologies and used in opto-electrical devices such as solar cells, displays, opto-electrical interfaces and circuitries. -

Synthesis and Characterisation of Delafossite Cufeo for Solar Energy
UPTEC Q 16006 Examensarbete 30 hp Juni 2016 Synthesis and characterisation of delafossite CuFeO2 for solar energy applications Axel Forslund Abstract Synthesis and characterisation of delafossite CuFeO2 for solar energy applications Axel Forslund Teknisk- naturvetenskaplig fakultet UTH-enheten Delafossite CuFeO2 is an intrinsic p-type semiconductor with a band gap around 1.5 eV. Further, it is composed of relatively abundant, nontoxic Besöksadress: elements, and therefor have potential to be an attractive material for solar Ångströmlaboratoriet Lägerhyddsvägen 1 energy harvesting.This Work examines three routes to synthesise this material. Hus 4, Plan 0 The first includes a sol-gel Deposition and then relies on solid state reaction above 650 Degrees Celsius in inert gas atmosphere. In this Work, no Delafossite Postadress: is obtained with this methoD.The second methoD is a hyDrothermal route to Box 536 751 21 Uppsala maKe particles under hyDrostatic pressure in an autoclave. Delafossite is obtained mixed with other phases.The thirD route includes aqueous Telefon: precipitation similar to the second route, but a temperature of 70 Degrees 018 – 471 30 03 Celsius and ambient pressure is sufficient to proDuce a pure Delafossite particle Telefax: phase. It provides a robust and simple way to maKe Delafossite CuFeO2 018 – 471 30 00 particles.The resulting particles are Deposited and compressed on glass into thin films.The films have a band gap slightly below 1.5 eV and show some Hemsida: photoactivity in electrochemical measurements. http://www.teknat.uu.se/student Handledare: Gerrit Boschloo Ämnesgranskare: Tomas Edvinsson Examinator: Åsa Kassman Rudolphi ISSN: 1401-5773, UPTEC Q 16006 Syntes och karakterisering av CuFeO2-delafossit för solenergitillämpningar Axel Forslund Som en del i att minska den mänskliga påverkan på klimatet måste användningen av fossila bränslen reduceras. -

DELAFOSSITE from KIMBERLY, NEVADA Ausrrwf
r02 rH E A M ERICAN M I N ERAINGIST heavy solution for separations of hydrous silicates readily decom- posed by acids. Possibly other analyses of zeolites exhibit irregu- larities due to similar substitutions. DELAFOSSITE FROM KIMBERLY, NEVADA AusrrwF. Rocnns,StanJoril Uni,aersi,ty Of the numerous copper minerals, one of the rarest and most interesting is delafossite,a cuprous metaferrite, with the formula, CuFeOzor CurO.FezOr. Delafossitewas first describedfrom the region of Ekaterinburg, Siberia, by Friedel in 1873.r No further mention of the mineral was made until 1913, when the writer2 called attention to its occurrenceat Bisbee, Arizona, and was able to establish it as a valid mineral species. Thq object of this note is to place on record the discovery of delafossite at a second American locality. A specimen of the mineral in question was sent to my colleague, professor W. F. Dietrich, by Mr. M. L. Crandall, Chief Engineer of the Con- solidated Coppermines Company. The specimenwas found on the 1200 ft. level of the Alpha mine (formerly called the Giroux) at Kimberly, Nevada. According to Mr. Crandall, ,,It was found in a body of clay containing some oxidized copper ore, occurring in oxidized jasperoid, near its contact with limestone.', Dr,scnrprrox oF THE Dcralossrra: The Kimberly delafossite is a black metallic mineral and occurs in spherical aggregatesfrom 1 to 3 mm. in diameter which are disseminated through a white to pale gray clay. (The clay is probably halloysite, the amorphous equivalent of crystalline kaolinite, as it is optically isotropic and has an index of refraction of 1 .543 + .003.) The spherulitic aggre- gates have a fairly well defined radial structure and hence may be called spherulites. -

Sample Journal Article Title Page Co-Authored Preprint
BNL-114168-2017-JA Electrochemical (de)lithiation of silver ferrite and composites: Mechanistic insights from ex-situ, in-situ, and operando x-ray techniques J. L. Durham, D. C. Bock Submitted to Physical Chemistry Chemical Physics August 2, 2017 Energy and Photon Sciences Directorate Brookhaven National Laboratory U.S. Department of Energy USDOE Office of Science (SC), Basic Energy Sciences (BES) (SC-22) Notice: This manuscript has been co-authored by employees of Brookhaven Science Associates, LLC under Contract No. DE-SC0012704 with the U.S. Department of Energy. The publisher by accepting the manuscript for publication acknowledges that the United States Government retains a non-exclusive, paid-up, irrevocable, world-wide license to publish or reproduce the published form of this manuscript, or allow others to do so, for United States Government purposes. DISCLAIMER This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, nor any of their contractors, subcontractors, or their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or any third party’s use or the results of such use of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof or its contractors or subcontractors. -

Structural and Magnetic Properties of Substituted Delafossite-Type Oxides Cucr1 − Xscxo2
World Journal of Condensed Matter Physics, 2013, 3, 1-8 1 doi:10.4236/wjcmp.2013.31001 Published Online February 2013 (http://www.scirp.org/journal/wjcmp) Structural and Magnetic Properties of Substituted Delafossite-Type Oxides CuCr1 − xScxO2 Taieb Elkhouni1, Mongi Amami1,2*, Pierre Strobel3, Abdelhamid Ben Salah1 1Laboratory of Material Sciences and Environnement, Faculty of Science of Sfax, Sfax, Tunisia; 2Unit of Material chemistry Re- search, University of Tunis Elmanar, Tunis, Tunisie; 3Neel Institute, CNRS, Grenoble, France. Email: *[email protected] Received July 28th, 2012; revised September 29th, 2012; accepted October 11th, 2012 ABSTRACT This work describes the scandium doping effect on the structural and magnetic properties of delafossite-type oxi- des CuCr1 − xScxO2. The lattice parameters were found to vary according to Vegard’s low. A reflection broadening is observed, that is ascribed to local lattice distortion due to the ionic radius difference between Cr3+ and the non-mag- netic dopants. Magnetic susceptibility measurements show that the dominant interactions are antiferromagnetic (AFM) but that doping induces significant changes. The coupling between the local spins at the Cr sites and do- ped metal transition may enhance spin fluctuations at the Cr sites, which break the residual magnetic degeneracy as fluctuation-induced symmetry breaking in a highly magnetic degenerate ground state manifold of some frustrated sys- tems. Keywords: Delafossite; XRD; Raman Spectroscopy; Magnetism 1. Introduction plications as transparent conductors [5]. Copper-scandium oxide (CSO) is a p-type transparent The family of layered oxides generally referred to as Cu+-based oxide with a delafossite structure. Holes in delafossites derive their name from the mineral CuFeO 2 CSO are introduced by substituting divalent species for [1], with which their crystal structures are isotypic. -

Delafossite» Observed for the First Time in Turkey
A NEW MINERAL «DELAFOSSITE» OBSERVED FOR THE FIRST TIME IN TURKEY Ahmet ÇAĞATAY and Oğuz ARDA Mineral Research and Exploration Institute of Turkey SUMMARY. — In association with some secondary minerals such as limonite, malachite, native copper, cuprite, tenorite and covelline; delafossite is found along the fractures of phyllitic rocks within the oxidized zone in the Malatya-Pötürge area. A detailed mineralogic study has been carried out about delafossite since it is observed first time in Turkey. INTRODUCTION A rock sample showing phyllite characteristics and containing a rarely encountered mineral delafossite is collected and brought for identification by Aykut Tümer (from the Geo- physical Department of the M.T.A.) from a locality to the south of Kılışık, near Çanakçı vil- lage, north of Pötürge (Malatya Province). Delafossite is found within the cracks of this phyllitic rock (Microphoto I), which underwent strong cataclastic deformation and silicification. The location where the sample is taken is shown in Figure I. Delafossite, which occurs in association with other secondary minerals, is studied under the ore microscope of Leitz-made Oftholux II Pol-Bk. In addition, reflection and Vickers mi- crohardness tests are carried out on the delafossite with the aid of a Vickers microscope. The reflection measurements are taken by using a Wolfram Carbide Standard of Zeiss-made, Stan- dard number: 474253, no. 061. The X-ray diffraction studies on delafossite are carried out by the aid of a Phillips X-ray diffractometer. DELAFOSSITE Chemical formula.— Ramdohr (I960) gives the chemical composition of delafossite as CuFeO2. Buist, Gadalla and White (1966), who carried out an experimental investigation on the Cu-Fe-O system, found that the natural delafossite resembles a synthetic phase of this sys- tem in 3Cu2O.Fe3O4 composition. -

Cu-Based Transparent Conducting Oxides Based on the Delafossite Structure
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1-1-2003 Cu-based transparent conducting oxides based on the delafossite structure Robert Bruce Gall Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Recommended Citation Gall, Robert Bruce, "Cu-based transparent conducting oxides based on the delafossite structure" (2003). Retrospective Theses and Dissertations. 19966. https://lib.dr.iastate.edu/rtd/19966 This Thesis is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Cu-based transparent conducting oxides based on the Delafossite structure by Robert Bruce Gall A dissertation submitted to the graduate faculty in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE Major: Materials Science and Engineering Program of Study Committee: David P. Cann, Major Professor Steve Martin Gordon Miller Iowa State University Ames, Iowa 2003 11 Graduate College Iowa State University This is to certify that the master's thesis of Robert Bruce Gall has met the thesis requirements for Iowa State University Signatures have been redacted for privacy TABLE OF CONTENTS CHAPTER 1. INTRODUCTION i 1.1 Background 1 1.1.1 Transparent Conducting Oxides