Influence of Different Polymeric Matrices on the Properties of Pentaerythritol Tetranitrate
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Greenchem Industries Chemical and Solvent Product List A-Z
GreenChem Industries Chemical And Solvent Product List A-Z 1,4-Butanediol (BDO) Formic Acid, 80%, 85%, 90%, 95% N-Butanol 2-Ethylhexanol (2-EH) Gamma Butyrolactone (GBL) N-Butyl Acetate (BUTAC) 2-Ethylhexyl Acrylate (2-EHA) Glacial Acetic Acid (GAA) N-Heptane 911P Glutaraldehyde 50% N-Methyl Pyrrolidone (NMP) Acetic Acid Glycerin USP K & Tech Grade N-Propanol Acetone Glycol Ether DB N-Propyl Acetate Acetonitrile Glycol Ether DE Neopentyl Glycol (NPG) Adipic Acid Glycol Ether DM Nitromethane Alpha-Methylstyrene (AMS) Glycol Ether DPM Nonyl Phenol Asphalt Cutback Glycol Ether DPM Acetate (DPM Acetate) NP-9, NP-10, NP-12 Benzoic Acid Glycol Ether DPnB Odorless Mineral Spirits (OMS) Benzyl Alcohol USP & TECH Glycol Ether DPnP Oxalic Acid 99.6% Benzyl Chloride Glycol Ether EB P-Chlorbenzotrifluoride (PCBTF) Boric Acid Glycol Ether EEP Perchloroethylene (PERC) Butyl Acrylate Glycol Ether EM Phenol 85%, 90%, 99% Caustic Potash 90% Glycol Ether EP Phthalic Anhydride Glycol Ether EPH Polyethylene Glycol (PEG) Caustic Soda Flakes & Beads Glycol Ether PM Propylene Carbonate (PC) Citric Acid, USP Kosher Glycol Ether PMA Propylene Glycol USP (PG USP K) Cyclohexane Glycol Ether PnB Propylene Glycol Industrial (PGI) Cyclohexylamine Glycol Ether PnP Sebacic Acid Cylcohexanone Glycol Ether TPM Secondary Butanol (SBA) D-Limonene Glycolic Acid 70% Sodium Laurel Sulfate (SLES) 28%, 60%, 70% DB Acetate GreenCool (Inhibited Glycols) Solv 100 Diacetone Alcohol (DAA) Hexane Solv 150 Dibasic Ester (DBE) Hexylene Glycol (HG) Solv 200 Dibutyl Phthalate (DBP) Hydrochloric -

Explosive Weapon Effectsweapon Overview Effects
CHARACTERISATION OF EXPLOSIVE WEAPONS EXPLOSIVEEXPLOSIVE WEAPON EFFECTSWEAPON OVERVIEW EFFECTS FINAL REPORT ABOUT THE GICHD AND THE PROJECT The Geneva International Centre for Humanitarian Demining (GICHD) is an expert organisation working to reduce the impact of mines, cluster munitions and other explosive hazards, in close partnership with states, the UN and other human security actors. Based at the Maison de la paix in Geneva, the GICHD employs around 55 staff from over 15 countries with unique expertise and knowledge. Our work is made possible by core contributions, project funding and in-kind support from more than 20 governments and organisations. Motivated by its strategic goal to improve human security and equipped with subject expertise in explosive hazards, the GICHD launched a research project to characterise explosive weapons. The GICHD perceives the debate on explosive weapons in populated areas (EWIPA) as an important humanitarian issue. The aim of this research into explosive weapons characteristics and their immediate, destructive effects on humans and structures, is to help inform the ongoing discussions on EWIPA, intended to reduce harm to civilians. The intention of the research is not to discuss the moral, political or legal implications of using explosive weapon systems in populated areas, but to examine their characteristics, effects and use from a technical perspective. The research project started in January 2015 and was guided and advised by a group of 18 international experts dealing with weapons-related research and practitioners who address the implications of explosive weapons in the humanitarian, policy, advocacy and legal fields. This report and its annexes integrate the research efforts of the characterisation of explosive weapons (CEW) project in 2015-2016 and make reference to key information sources in this domain. -

High Performance Ester Plasticizers
High Performance Ester Plasticizers Abstract Traditional elastomer polymers, such as nitrile, polychloroprene, chlorinated polyethylene and chlorosulfonated polyethylene, have for years used moderate- to low- performance ester plasticizers. However, longevity requirements for rubber articles made from these elastomers have created a need for higher-performance ester plasticizers. With the increasing high-temperature demands required by automotive, other elastomers such as acrylic, high-saturated nitrile, epichlorohydrin and ethylene propylene diene monomer EPDM are replacing the more traditional elastomers. Plasticizers commonly used for the traditional and the high-temperature polymers are extractable, incompatible or too volatile. This paper provides information on plasticizers that are designed for traditional elastomers and high-performance polymers. The test data will include heat aging, extraction by hydrocarbons and low-temperature as molded after aging. The information provided indicates that the permanence of the plasticizer after these various agings is the key to the retention of physical properties. Introduction End uses for elastomer compounds are quite diverse, but they can be loosely categorized as being either general performance or higher performance applications. Each of these performance categories requires a different set of considerations in terms of compounding with ester plasticizers. An ester plasticizer, in its simplest concept, is a high-boiling organic solvent that when added to an elastomeric polymer reduces stiffness and permits easier processing.1 For general performance applications, compounders require moderate performance in several areas without particular emphasis on any one. Some general performance ester plasticizers used in the marketplace today are DOA, DIDA, DIDP, DOP, DINP and other phthalates and adipates made from straight-chain alcohols of 7–11 carbons in length. -

Surface Modification of Engineering Plastics Through Swelling In
#2008 The Society of Polymer Science, Japan Surface Modification of Engineering Plastics through Swelling in Supercritical Carbon Dioxide By Toshimi TAKAJO,1;2 Atsushi TAKAHARA,1;Ã and Takefumi KICHIKAWA2 In order to improve the tribological characteristics of polymer materials, the authors tried to impregnate the surface of engineering plastics with the lubricating oil by using supercritical carbon dioxide. The oil impregnation ratio on crystalline polymer was influenced by both the glass transition temperature (Tg) and the degree of crystallinity. In case of using the polymer possessing lower Tg with large difference between the Tg and treatment temperature, the higher impregnation ratio was obtained, and the crystallite in crystalline polymer prevented the impregnation of lubricating oil. The results of polarized optical microscopic observations and FT-IR spectroscopy studies indicated that the lubricating oil was preferentially impregnated to the amorphous region in crystalline polymer, and the high-concentration layer of lubricating oil having the thickness of ca. 30 mm was formed at the vicinity of surface area. The result obtained in this study, which reports the preferential lubricating oil impregnation to the surface of crystalline polymer under supercritical carbon dioxide, suggests that the tribological characteristics of crystalline polymer would be improved by applying this oil impregnation method without sacrificing the bulk mechanical strength. KEY WORDS: Supercritical Carbon Dioxide / Oil Impregnation / Engineering Plastic / Tribology / Crystallinity / Many studies carried out by utilizing supercritical fluid have lubricating oil at high temperature over long periods of time to been recently reported, and the representative examples of the impregnate the product with lubricating oil. According to such studies are as follows: researches for the polymerization the method, however, it is unavoidable that the deterioration of reaction in supercritical fluid, the supercritical extraction- polymer is accelerated. -

Raw Materials, Chemicals and Additives Handbook – March 2009 Product Listings
Raw Materials, Chemicals and Additives Handbook – March 2009 Product Listings A. ADDITIVES AND CHEMICAL SPECIALTIES 13350 Polyfunctional Aziridines 10100 Accelerating and Vulcanizing Agents: 13400 Polyols 10150 Abrasives 13450 Resorcinol Resins 10200 Dithiocarbamates 13500 Silane 10250 Sulfur 13550 Silicone 10300 Thiazoles 13600 Toluene Diisocyanate (TDI) 10350 Thiuram Sulfides 13650 Urea-Formaldehyde Resins 10400 Zinc Oxides 13700 Zinc Salts 10450 Acids (Non-Fatty) 13750 Zirconium 10500 Additives 13800 Defoamers: 10550 Adhesion Promoters: 13850 Aluminum Stearate 10600 Adhesive Bonding Primers 13900 Amyl Alcohol 10650 Alpha Methyl-Styrene Polymers 13950 Capryl Alcohol 10700 Hydrogenated Resins 14000 Castor Oil 10750 Pentaerythritol Esters 14050 Corn Oil 10800 Phenolic Resins 14100 Decyl Alcohol 10850 Resorcinol 14150 Diethylene Glycol Monolaurate 10900 Silane 14200 Glyceryl Monostearate 10950 Silicone 14250 Mineral Oil 11000 Unsaturated Polyesters 14300 Nonyl Alcohol 11050 Vinyl Pyridine Monomer 14350 Octyl Alcohol 11100 Amine Neutralizers 14400 Palmitic Acid 11150 Ammonia 14450 Pine Oil 11200 Anti-Foaming Agents: 14500 Polyalkyl Glycol 11250 Non-Silicone 14550 Silicone Oils 11300 Silicone 14600 Stearic Acid 11350 Antioxidants: 14650 Sulfonic Acid Salts 11400 Phenolic 14700 Tri-Butyl Citrate 11450 Phosphite 14750 Tri-Butyl Phosphate 11500 Anti-Settling Agents 14800 Turkey Red Oil 11550 Anti-Skinning Agents 14900 Dispensing Agents 11600 Anti-Static Chemicals 14950 Dispersing Agents, see 22900 Surfactants and 11650 Anti-Tack Agents Dispersing -

Guide for the Selection of Commercial Explosives Detection Systems For
2.5.3.8 EXPRAY Field Test Kit EXPRAY is a unique, aerosol-based field test kit for the detection of what the manufacturer refers to as Group A explosives (TNT, DNT, picric acid, etc.), Group B explosives (Semtex H, RDX, PETN, NG, smokeless powder, etc.), and compounds that contain nitrates that are used in improvised explosives. Detection of explosive residue is made by observing a color change of the test paper. EXPRAY can be used in a variety of applications, and although in some aspects it does not perform as well as many of the other trace detectors discussed in this section, it costs only $250. This very low cost, coupled with simplicity and ease of use, may make it of interest to many law enforcement agencies (see the EXPRAY kit in fig. 13). The EXPRAY field kit2 is comprised of the following items: - one can of EXPRAY-1 for Group A explosives, - one can of EXPRAY-2 for Group B explosives, - one can of EXPRAY-3 for nitrate-based explosives (ANFO, black powder, and commercial and improvised explosives based on inorganic nitrates), - special test papers which prevent cross contamination. Figure 13. Photo of the EXPRAY Field Test Kit for explosives Initially, a suspected surface (of a package, a person’s clothing, etc.) is wiped with the special test paper. The paper is then sprayed with EXPRAY-1. The appearance of a dark violet-brown color indicates the presence of TNT, a blue-green color indicates the presence of DNT, and an orange color indicates the presence of other Group A explosives. -
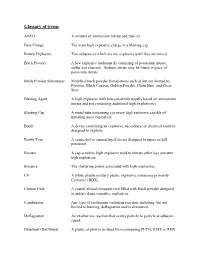
TWGFEX Glossary of Terms
Glossary of terms ANFO A mixture of ammonium nitrate and fuel oil. Base Charge The main high explosive charge in a blasting cap. Binary Explosive Two substances which are not explosive until they are mixed. Black Powder A low explosive traditionally consisting of potassium nitrate, sulfur and charcoal. Sodium nitrate may be found in place of potassium nitrate. Black Powder Substitutes Modified black powder formulations such as but not limited to: Pyrodex, Black Canyon, Golden Powder, Clean Shot, and Clear Shot. Blasting Agent A high explosive with low-sensitivity usually based on ammonium nitrate and not containing additional high explosive(s). Blasting Cap A metal tube containing a primary high explosive capable of initiating most explosives. Bomb A device containing an explosive, incendiary, or chemical material designed to explode. Booby Trap A concealed or camouflaged device designed to injure or kill personnel. Booster A cap sensitive high explosive used to initiate other less sensitive high explosives. Brisance The shattering power associated with high explosives. C4 A white pliable military plastic explosive containing primarily Cyclonite (RDX). Cannon Fuse A coated, thread-wrapped cord filled with black powder designed to initiate flame-sensitive explosives. Combustion Any type of exothermic oxidation reaction, including, but not limited to burning, deflagration and/or detonation. Deflagration An exothermic reaction that occurs particle to particle at subsonic speed. Detasheet (Det Sheet) A plastic explosive in sheet form containing PETN, HMX or RDX. Detonation An exothermic reaction that propagates a shockwave through an explosive at supersonic speed (greater than 3300ft/sec). Detonation Cord (Det-Cord) A plastic/fiber wrapped cord containing a core of PETN or RDX. -

Review of Exposure and Toxicity Data for Phthalate Substitutes
EXECUTIVE SUMMARY In August 2008, the U.S. Congress passed the Consumer Product Safety Improvement Act of 2008 (CPSIA) placing restrictions on the use of six dialkyl ortho-phthalates (o- DAPs) in children’s toys or child care articles. The CPSIA also directs the Consumer Product Safety Commission (CPSC) to convene a Chronic Hazard Advisory Panel to investigate the potential health effects of phthalates and phthalate substitutes. The purpose of this report is to identify o-DAP substitutes that are currently being used in children’s articles, or are probable future candidates, and to summarize the potential human health risks associated with using these chemicals in this manner. Chemicals were identified as the most likely alternatives to o-DAPs in children’s articles based on a variety of factors which included their compatibility with polyvinyl chloride (PVC). The five chemicals identified by this report as the most likely o-DAP alternatives are acetyl tri-n-butyl citrate (ATBC), di(2-ethylhexyl) adipate (DEHA), 1,2- cyclohexanedicarboxylic acid, dinonyl ester (DINCH), trioctyltrimellitate (TOTM),and di(2-ethylhexyl) terephthalate (DEHT or DOTP). All, except TOTM, have been cited as already being used in children’s articles. However, TOTM is compatible with PVC – the most popular resin for children’s soft plastic toys and other articles – and thus a likely o- DAP alternative. The review of the potential risks of using these chemicals in children’s articles focused on the amount and quality of data available for the chemical. Key parameters included physical-chemical properties, migration rates, and all available exposure, hazard, and dose-response information. -

Chemical Compatibility Chart
Chemical Compatibility Chart 1 Inorganic Acids 1 2 Organic acids X 2 3 Caustics X X 3 4 Amines & Alkanolamines X X 4 5 Halogenated Compounds X X X 5 6 Alcohols, Glycols & Glycol Ethers X 6 7 Aldehydes X X X X X 7 8 Ketone X X X X 8 9 Saturated Hydrocarbons 9 10 Aromatic Hydrocarbons X 10 11 Olefins X X 11 12 Petrolum Oils 12 13 Esters X X X 13 14 Monomers & Polymerizable Esters X X X X X X 14 15 Phenols X X X X 15 16 Alkylene Oxides X X X X X X X X 16 17 Cyanohydrins X X X X X X X 17 18 Nitriles X X X X X 18 19 Ammonia X X X X X X X X X 19 20 Halogens X X X X X X X X X X X X 20 21 Ethers X X X 21 22 Phosphorus, Elemental X X X X 22 23 Sulfur, Molten X X X X X X 23 24 Acid Anhydrides X X X X X X X X X X 24 X Represents Unsafe Combinations Represents Safe Combinations Group 1: Inorganic Acids Dichloropropane Chlorosulfonic acid Dichloropropene Hydrochloric acid (aqueous) Ethyl chloride Hydrofluoric acid (aqueous) Ethylene dibromide Hydrogen chloride (anhydrous) Ethylene dichloride Hydrogen fluoride (anhydrous) Methyl bromide Nitric acid Methyl chloride Oleum Methylene chloride Phosphoric acid Monochlorodifluoromethane Sulfuric acid Perchloroethylene Propylene dichloride Group 2: Organic Acids 1,2,4-Trichlorobenzene Acetic acid 1,1,1-Trichloroethane Butyric acid (n-) Trichloroethylene Formic acid Trichlorofluoromethane Propionic acid Rosin Oil Group 6: Alcohols, Glycols and Glycol Ethers Tall oil Allyl alcohol Amyl alcohol Group 3: Caustics 1,4-Butanediol Caustic potash solution Butyl alcohol (iso, n, sec, tert) Caustic soda solution Butylene -

United States Patent (19) 11 Patent Number: 5,009,728 Chan Et Al
United States Patent (19) 11 Patent Number: 5,009,728 Chan et al. (45. Date of Patent: Apr. 23, 1991 (54 CASTABLE, INSENSITIVE ENERGETIC 4,141,910 2/1979 Flanagan et al. ..................... 149/88 COMPOSITIONS 4,325,759 4/1982 Voigt et al. ..... - 49/19.92 4,361,526 11/1982 Allen ....................................... 264/3 (75) Inventors: May L. Chan; Alan D. Turner, both of 4,393,199 7/1983 Manser ................ ... 149/19.6 Ridgecrest, Calif. 4,426,540 1/1984 Shackelford et al. 149/19.91 4,456,494 6/1984 Maes et al. ........... ... 149/2 73) Assignee: The United States of America as 4,555,277 11/1985 Scribner ....... 149/19.4 represented by the Secretary of the 4,632,714 12/1986 Abegget al. ........................... 149/2 Navy, Washington, D.C. 4,764,316 8/1988 Brown et al..... ... 264/3.1 4,806,613 2/1989 Wardle......... ... 149/19.6 21 Appl. No.: 464,076 4,889,571 12/1989 Willer et al. ....................... 149/19.9 22 Filed: Jan. 12, 1990 OTHER PUBLICATIONS 51 int.C.'.............................................. C06B 45/10 52 U.S. C. ........................ ....... 149/19.1; 149/19.4; P. A. Mancinelli and S. O. Norris, "Macromonomers 149/19.5; 149/19.6; 149/19.9; 149/19.91; Lead to Acrylic Hot Melt and Solvent PSAs", reprint 149/88; 149/92 from Adhesives Age, Sep. 1985, (copy enclosed). (58) Field of Search .................... 149/19.4, 19.6, 199, Primary Examiner-Edward A. Miller 149/19.91, 19.1, 19.5, 88, 92 Attorney, Agent, or Firm-Donald E. -

Theoretical Principles for Successful Littoral Special Operations
Calhoun: The NPS Institutional Archive Theses and Dissertations Thesis and Dissertation Collection 2016-06 Maritime SOF in the littorals: theoretical principles for successful littoral special operations Grimeland, Torbjorn Monterey, California: Naval Postgraduate School http://hdl.handle.net/10945/49475 NAVAL POSTGRADUATE SCHOOL MONTEREY, CALIFORNIA THESIS MARITIME SOF IN THE LITTORALS: THEORETICAL PRINCIPLES FOR SUCCESSFUL LITTORAL SPECIAL OPERATIONS by Torbjorn Grimeland Oscar van der Veen June 2016 Thesis Advisor: Kalev I. Sepp Second Reader: Ian Rice Approved for public release; distribution is unlimited THIS PAGE INTENTIONALLY LEFT BLANK REPORT DOCUMENTATION PAGE Form Approved OMB No. 0704-0188 Public reporting burden for this collection of information is estimated to average 1 hour per response, including the time for reviewing instruction, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden, to Washington headquarters Services, Directorate for Information Operations and Reports, 1215 Jefferson Davis Highway, Suite 1204, Arlington, VA 22202-4302, and to the Office of Management and Budget, Paperwork Reduction Project (0704-0188) Washington DC 20503. 1. AGENCY USE ONLY 2. REPORT DATE 3. REPORT TYPE AND DATES COVERED (Leave blank) June 2016 Master’s thesis 4. TITLE AND SUBTITLE 5. FUNDING NUMBERS MARITIME SOF IN THE LITTORALS: THEORETICAL PRINCIPLES FOR SUCCESSFUL LITTORAL SPECIAL OPERATIONS 6. AUTHOR(S) Torbjorn Grimeland and Oscar van der Veen 7. PERFORMING ORGANIZATION NAME(S) AND ADDRESS(ES) 8. PERFORMING Naval Postgraduate School ORGANIZATION REPORT Monterey, CA 93943-5000 NUMBER 9. -

Chapter Eleven Convention on the Marking of Plastic
CHAPTER ELEVEN CONVENTION ON THE MARKING OF PLASTIC EXPLOSIVES FOR THE PURPOSE OF DETECTION 1991 (‘Explosives Convention’) 1. This Convention is very different from the other eleven, even the Tokyo Convention, in that it is not directed at the arrest and prosecution of terrorists, but at better detection of a particularly dangerous explosive used by terrorists and other criminals. It therefore does not create any offences, though the implementing legislation will have to. The basic purpose of the Convention is to prevent terrorists, or for that matter any other person with no legitimate right to them, being able to obtain unmarked plastic explosives. The existence of criminal penalties may help to deter crime, but the increased possibility of detection should be an even more effective deterrent. Most States have rigorous controls on explosives, including their manufacture, possession, transport and use. The main thrust of the Convention is therefore to tackle the problem posed by the difficulty of detecting unmarked plastic explosives. The Convention does not therefore follow the structure or substance of the other conventions. The United Nations lists the Convention as one of the 12 counter-terrorism conventions only because of its importance for the continual fight against terrorism. 2. The sabotage of civil aircraft by terrorists grew markedly in the 1980s. In 1985 there were 13 acts of sabotage killing 473 people.149 In 1989 two acts killed 279, and it was that year, in the aftermath of what had by then been the single most serious international terrorist attack, that the Convention was conceived. On 21 December 1988 a Boeing 747 aircraft of Pan American Airways on flight PA103 exploded over Scotland killing all 259 passengers and crew and eleven residents of Lockerbie.