(Information) Gap: the Importance of Exploration and Discovery For
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Selection, Constraint, and Adaptation in the Visual Genes of Neotropical Cichlid Fishes and Other Vertebrates
SELECTION, CONSTRAINT, AND ADAPTATION IN THE VISUAL GENES OF NEOTROPICAL CICHLID FISHES AND OTHER VERTEBRATES by Frances Elisabeth Hauser A thesis submitted in conformity with the requirements for the degree of Doctor of Philosophy Ecology and Evolutionary Biology University of Toronto © Copyright by Frances E. Hauser 2018 SELECTION, CONSTRAINT, AND ADAPTATION IN THE VISUAL GENES OF NEOTROPICAL CICHLID FISHES AND OTHER VERTEBRATES Frances E. Hauser Doctor of Philosophy, 2018 Department of Ecology and Evolutionary Biology University of Toronto 2018 ABSTRACT The visual system serves as a direct interface between an organism and its environment. Studies of the molecular components of the visual transduction cascade, in particular visual pigments, offer an important window into the relationship between genetic variation and organismal fitness. In this thesis, I use molecular evolutionary models as well as protein modeling and experimental characterization to assess the role of variable evolutionary rates on visual protein function. In Chapter 2, I review recent work on the ecological and evolutionary forces giving rise to the impressive variety of adaptations found in visual pigments. In Chapter 3, I use interspecific vertebrate and mammalian datasets of two visual genes (RH1 or rhodopsin, and RPE65, a retinoid isomerase) to assess different methods for estimating evolutionary rate across proteins and the reliability of inferring evolutionary conservation at individual amino acid sites, with a particular emphasis on sites implicated in impaired protein function. ii In Chapters 4, and 5, I narrow my focus to devote particular attention to visual pigments in Neotropical cichlids, a highly diverse clade of fishes distributed across South and Central America. -

Two New Species of Australoheros (Teleostei: Cichlidae), with Notes on Diversity of the Genus and Biogeography of the Río De La Plata Basin
Zootaxa 2982: 1–26 (2011) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2011 · Magnolia Press ISSN 1175-5334 (online edition) Two new species of Australoheros (Teleostei: Cichlidae), with notes on diversity of the genus and biogeography of the Río de la Plata basin OLDŘICH ŘÍČAN1, LUBOMÍR PIÁLEK1, ADRIANA ALMIRÓN2 & JORGE CASCIOTTA2 1Department of Zoology, Faculty of Science, University of South Bohemia, Branišovská 31, 370 05, České Budějovice, Czech Republic. E-mail: [email protected], [email protected] 2División Zoología Vertebrados, Facultad de Ciencias Naturales y Museo, UNLP, Paseo del Bosque, 1900 La Plata, Argentina. E-mail: [email protected], [email protected] Abstract Two new species of Australoheros Říčan and Kullander are described. Australoheros ykeregua sp. nov. is described from the tributaries of the río Uruguay in Misiones province, Argentina. Australoheros angiru sp. nov. is described from the tributaries of the upper rio Uruguai and middle rio Iguaçu in Brazil. The two new species are not closely related, A. yke- regua is the sister species of A. forquilha Říčan and Kullander, while A. angiru is the sister species of A. minuano Říčan and Kullander. The diversity of the genus Australoheros is reviewed using morphological and molecular phylogenetic analyses. These analyses suggest that the described species diversity of the genus in the coastal drainages of SE Brazil is overestimated and that many described species are best undestood as representing cases of intraspecific variation. The dis- tribution patterns of Australoheros species in the Uruguay and Iguazú river drainages point to historical connections be- tween today isolated river drainages (the lower río Iguazú with the arroyo Urugua–í, and the middle rio Iguaçu with the upper rio Uruguai). -

Apistogramma Ortegai (Teleostei: Cichlidae), a New Species of Cichlid Fish from the Ampyiacu River in the Peruvian Amazon Basin
Zootaxa 3869 (4): 409–419 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2014 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3869.4.5 http://zoobank.org/urn:lsid:zoobank.org:pub:CB38DF91-EC70-4B17-9B9A-18C949431C1D Apistogramma ortegai (Teleostei: Cichlidae), a new species of cichlid fish from the Ampyiacu River in the Peruvian Amazon basin RICARDO BRITZKE1, CLAUDIO OLIVEIRA1 & SVEN O. KULLANDER2 1Universidade Estadual Paulista, Instituto de Biociências, Departamento de Morfologia, Rubião Jr. s/n. CEP 18618-970. Botucatu, SP, Brazil. E-mail: [email protected] 2Department of Zoology, Swedish Museum of Natural History, PO Box 50007, SE-104 05 Stockholm, Sweden Abstract Apistogramma ortegai, new species, is described from small streams tributaries of the Ampiyacu River near Pebas, in east- ern Peru. It belongs to the Apistogramma regani species group and is distinguished from all other species of Apistogramma by the combination of contiguous caudal spot to bar 7, presence of abdominal stripes, short dorsal-fin lappets in both sexes, absence of vertical stripes on the caudal fin, and reduced number of predorsal and prepelvic scales. Key words: Geophaginae, Geophagini, Amazonia, Freshwater, Morphology, Taxonomy Resumen Apistogramma ortegai, nueva especie, es descrita desde pequeños tributario del rio Ampiyacu cerca de Pebas, en el este del Perú. Pertenece al grupo de especies de A. regani y es distinguido de todas las otras especies de Apistograma por la combinación de la barra 7 conectada con una mancha en la aleta caudal, presencia de líneas abdominales, membranas de la aleta dorsal cortas en ambos sexos, ausencia de líneas verticales en la aleta caudal, y reducido número de escamas pre- dorsales y prepélvicas. -

06 Staeck Final Version 1.Indd
Zoologische Abhandlungen (Dresden) 56: 991–971–97 91 Geophagus gottwaldi sp. n. - a new species of cichlid fi sh (Teleostei: Perciformes: Cichlidae) from the drainage of the upper río Orinoco in Venezuela INGO SCHINDLER 1 & WOLFGANG STAECK 2 1 Warthestr. 53a, D-12051 Berlin 2 Auf dem Grat 41a, D-14195 Berlin Abstract. Geophagus gottwaldi sp. n. is described from the drainage of the upper río Orinoco in the Estado Amazonas in southwestern Venezuela. It can be distinguished from all other described Geophagus species by the following combination of characters: a prominent dark infraorbital stripe, caudal fi n with a pattern of roundish light spots, a rectangular midlateral spot, 34–36 scales in a lateral line and total length of more than 20 cm. Resumo. Geophagus gottwaldi, espécie nova, é descrita da drenagem do alto rio Orinoco (Estado Amazonas, Venezuela). Geophagus gottwaldi é distinta das demais espécies descritas do gênero Geophagus pela combinação das seguintes caracteristicas: faixa intraorbital completa, nadadeira caudal com manchas claras arredondadas, uma grande mancha rectangular preta no meio de corpo, 34–36 escamas no linha lateral e tamanho grande (TL > 20 cm). Resumen. Se describe una nueva especie de cíclido, Geophagus gottwaldi, de la cuenca del alto río Orinoco (Estado Amazonas de Venezuela). La nueva especie se distingue de todas las demás especies del género Geophagus por la siguiente combinación de carácteres diagnósticos: una banda oscura conspicua intraorbital que extiende desde el ojo hasta el ángulo del preopérculo, aleta caudal con manchas blancas redondas, una grande mancha rectangular en el centro del cuerpo, 34–36 escamas en la serie longitudinal y tamaño grande (TL >20 cm). -

Apistogramma Psammophila – a New Geophagine Dwarf Cichlid (Teleostei: Cichlidae) from the Rio Atabapo Drainage in Colombia and Venezuela
69 (1): 103 –110 © Senckenberg Gesellschaft für Naturforschung, 2019. 27.2.2019 Apistogramma psammophila – a new geophagine dwarf cichlid (Teleostei: Cichlidae) from the Rio Atabapo drainage in Colombia and Venezuela Wolfgang Staeck 1 & Ingo Schindler 2 1 [email protected] — 2 [email protected] Submitted June 29, 2018. Accepted January 10, 2019. Published online at www.senckenberg.de/vertebrate-zoology on February 15, 2019. Published in print on February 27, 2019. Editor in charge: Uwe Fritz Abstract Apistogramma psammophila sp. n. is described from the lower Rio Atabapo drainage in Colombia (Departamento Guainía) and Venezuela (Estado Amazonas). It can be distinguished from all congeners by the following combination of characters: Two dark horizontal stripes on ERG\VLGHWZRSHFWRUDOVSRWVVXERUELWDOVWULSHEHFRPLQJPXFKZLGHUYHQWUDOO\DQGQRFDXGDOVSRW,QDGXOWPDOHVVRIWGRUVDODQGDQDO¿QV HDFKZLWKORQJ¿ODPHQWRXVSURORQJDWLRQDOPRVWUHDFKLQJSRVWHULRUPDUJLQRIFDXGDO¿QDQGSHOYLF¿QVZLWK¿ODPHQWRXVH[WHQVLRQQHDUO\ UHDFKLQJPLGGOHRIDQDO¿QEDVH It is most similar to Apistogramma diplotaenia Kullander, 1987, but differs from this species by an irregular pattern of 6 – 8 short verti- cal dark bar-like markings below the lower lateral stripe, and adult males with reddish area on cheek and chest between eye and pectoral D[LOODDQGGLVWLQFWO\ODQFHRODWHFDXGDO¿Q. Resumen Apistogramma psammophila sp. n. se describe a partir de la cuenca del río Atabapo bajo en Colombia (Departamento Guainía) y Venezuela (Estado Amazonas). La nueva especie se distingue de todas las demás -

2010 Board of Governors Report
American Society of Ichthyologists and Herpetologists Board of Governors Meeting Westin – Narragansett Ballroom B Providence, Rhode Island 7 July 2010 Maureen A. Donnelly Secretary Florida International University College of Arts & Sciences 11200 SW 8th St. - ECS 450 Miami, FL 33199 [email protected] 305.348.1235 13 June 2010 The ASIH Board of Governor's is scheduled to meet on Wednesday, 7 July 2010 from 5:00 – 7:00 pm in the Westin Hotel in Narragansett Ballroom B. President Hanken plans to move blanket acceptance of all reports included in this book that cover society business for 2009 and 2010 (in part). The book includes the ballot information for the 2010 elections (Board of Governors and Annual Business Meeting). Governors can ask to have items exempted from blanket approval. These exempted items will be acted upon individually. We will also act individually on items exempted by the Executive Committee. Please remember to bring this booklet with you to the meeting. I will bring a few extra copies to Providence. Please contact me directly (email is best - [email protected]) with any questions you may have. Please notify me if you will not be able to attend the meeting so I can share your regrets with the Governors. I will leave for Providence (via Boston on 4 July 2010) so try to contact me before that date if possible. I will arrive in Providence on the afternoon of 6 July 2010 The Annual Business Meeting will be held on Sunday 11 July 2010 from 6:00 to 8:00 pm in The Rhode Island Convention Center (RICC) in Room 556 AB. -
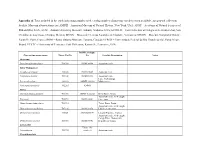
Appendix A. Taxa Included in the Study Indicating Samples Used, Catalog Number of Museum Vouchers When Available, and General Collection Locality
Appendix A. Taxa included in the study indicating samples used, catalog number of museum vouchers when available, and general collection locality. Museum abbreviations are: AMNH – American Museum of Natural History, New York, USA; ANSP – Academy of Natural Sciences of Philadelphia, USA; AUM – Auburn University Museum, Auburn, Alabama, USA; ECOSUR – Fish Collection at Colegio de la Frontera Sur, San Cristóbal de Las Casas, Chiapas, Mexico; MCNG – Museo de Ciencias Naturales de Guanare, Venezuela; MNHN – Muséum National d’Histoire Naturelle, Paris, France; ROM – Royal Ontario Museum, Toronto, Canada; UFRGS – Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil; UTFTC – University of Tennessee Fish Collection, Knoxville, Tennessee, USA. ROM Catalogue Current taxonomy name Tissue Cat No No Locality Description Notes Outgroup Pseudetroplus maculatus T14743 ROM 98998 Aquarium trade India-Madagascar Etroplus suratensis T13505 ROM 93809 Aquarium trade Paratilapia polleni T13100 ROM 88333 Aquarium trade Lake Andrapongy, Paretroplus damii 201936 AMNH 201936 Madagascar Paretroplus polyactis T12265 AMNH Africa Chromidotilapia guntheri T11700 AMNH I-226361 Beffa River, Benin Aquarium trade, wild caught, Etia nguti T10792 ROM 88042 Cameroon Hemichromis bimaculatus T11719 Tchan Duga, Benin Aquarium trade, wild caught, Heterochromis multidens T07136 ROM 88350 Lobeke, Cameroon Oreochromis niloticus 9092S AMNH254194 Littoral Province, Guinea Aquarium trade, wild caught, Congo River, Democratic Orthochromis stormsi T10766 ROM 88041 Republic of -

Curriculum Vitae, October 26, 2020
H. López-Fernández, Curriculum Vitae, October 26, 2020 HERNÁN LÓPEZ-FERNÁNDEZ - CURRICULUM VITAE Department of Ecology and Evolutionary Biology and Program in the Environment, University of Michigan, 1105 N. University, Biological Sciences Building, Office 2014, Ann Arbor, MI 48109, USA Email: [email protected] - Phone: Office - (734) 764-4816 Web: https://sites.google.com/site/hlffishes/ Professional preparation: 2006 – 2007 - Postdoctoral Research Associate, Section of Ecology, Evolution and Systematic Biology, Texas A&M University. Postdoctoral advisors: Kirk O. Winemiller and Rodney L. Honeycutt. 2005 – 2006 - Postodoctoral Fellow, Section of Integrative Biology, University of Texas at Austin. Postdoctoral advisor: Daniel I. Bolnick. 2004 - Ph. D. Section of Ecology, Evolution and Systematic Biology, Texas A&M University. Dissertation Title: Phylogeny of geophagine cichlids from South America (Perciformes: Cichlidae). Kirk O. Winemiller and Rodney L. Honeycutt, Dissertation Co-Advisors. 1998 - Licenciate in Biology (B.S.). Universidad de Los Andes, Mérida, Venezuela. Major: Biology, Minor (Specialization): Animal Ecology. Professional appointments: 2020-2023 – Associate Chair for Collections, EEB Museum of Zoology and Herbarium, Department of Ecology and Evolutionary Biology, University of Michigan. 2019-2024 – Research Associate, Division of Ichthyology, Department of Natural History, Royal Ontario Museum, Toronto, Canada. 2019 – Affiliated Faculty, Michigan Institute for Data Science (MIDAS), University of Michigan, Ann Arbor, USA. -

The Guiana Shield
THIRTEEN The Guiana Shield NATHAN K. LUJAN and JONATHAN W. ARMBRUSTER Highland areas that serve as sources and boundaries for the a superfamily sister to all other Siluriformes, and their bio- great rivers of South America can be broadly divided into two geographic tractability due to distributions across headwater categories based on their geologic age and origin. As reviewed habitats and associated allopatric distribution patterns among elsewhere in this volume (Chapters 15 and 16), the allochtho- sister taxa. We conclude that the diverse loricariid fauna of the nous terrains and massive crustal deformations of the Andes Guiana Shield accumulated gradually over tens of millions of Mountains that comprise the extremely high-elevation west- years with major lineages being shaped by geologic evolution ern margin of South America have their origins in diastrophic across the whole continent, and not as the result of a rapid, (distortional) tectonic activity largely limited to the Late Paleo- geographically restricted adaptive radiation. We demonstrate gene and Neogene (<25 Ma; Gregory-Wodzicki 2000). In con- the role of the Guiana and Brazilian shields as ancient reser- trast, vast upland regions across much of the interior of the voirs of high-gradient lotic habitats infl uencing the origin of continent have been relatively tectonically quiescent since the frequently rheophilic loricariid taxa. We also show how diver- Proterozoic (>550 Ma; Gibbs and Baron 1993) and exhibit a sifi cation was infl uenced by a restricted number of landscape topography that is instead largely the result of nondeforma- scale features: especially dispersal and vicariance across several tional, epeirogenic uplift of the Guiana and Brazilian shields geologically persistent corridors, expansion and contraction of and subsequent erosion of overlying sedimentary formations. -

Exon-Based Phylogenomics Strengthens the Phylogeny of Neotropical Cichlids And
bioRxiv preprint doi: https://doi.org/10.1101/133512; this version posted July 13, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Exon-based phylogenomics strengthens the phylogeny of Neotropical cichlids and 2 identifies remaining conflicting clades (Cichliformes: Cichlidae: Cichlinae) 3 4 Katriina L. Ilves1,2*, Dax Torti3,4, and Hernán López-Fernández1 5 1 Department of Natural History, Royal Ontario Museum, 100 Queen’s Park, Toronto, 6 ON M5S 2C6 Canada 7 2 Current address: Biology Department, Pace University, 1 Pace Plaza, New York, NY 8 10038 9 3 Donnelly Sequencing Center, University of Toronto, 160 College Street, Toronto, ON 10 M5S 3E1 Canada 11 4 Current address: Ontario Institute for Cancer Research, MaRS Center, 661 University 12 Avenue, Toronto, ON M5G 0A3 Canada 13 *Corresponding author at: Biology Department, Pace University, 1 Pace Plaza, New 14 York, NY 10038; email: [email protected] 15 16 Abstract 17 The phenotypic, geographic, and species diversity of cichlid fishes have made them a 18 group of great interest for studying evolutionary processes. Here we present a targeted- 19 exon next-generation sequencing approach for investigating the evolutionary 20 relationships of cichlid fishes (Cichlidae), with focus on the Neotropical subfamily 21 Cichlinae using a set of 923 primarily single-copy exons designed through mining of the 22 Nile tilapia (Oreochromis niloticus) genome. Sequence capture and assembly were 23 robust, leading to a complete dataset of 415 exons for 139 species (147 terminals) that 1 bioRxiv preprint doi: https://doi.org/10.1101/133512; this version posted July 13, 2017. -

Exon-Based Phylogenomics Strengthens the Phylogeny of Neotropical Cichlids And
bioRxiv preprint doi: https://doi.org/10.1101/133512; this version posted May 2, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Exon-based phylogenomics strengthens the phylogeny of Neotropical cichlids and 2 identifies remaining conflicting clades (Cichlomorphae: Cichlidae: Cichlinae) 3 4 Katriina L. Ilves1,2*, Dax Torti3,4, and Hernán López-Fernández1 5 1 Department of Natural History, Royal Ontario Museum, 100 Queen’s Park, Toronto, 6 ON M5S 2C6 Canada 7 2 Current address: Biology Department, Pace University, 1 Pace Plaza, New York, NY 8 10038 9 3 Donnelly Sequencing Center, University of Toronto, 160 College Street, Toronto, ON 10 M5S 3E1 Canada 11 4 Current address: Ontario Institute for Cancer Research, MaRS Center, 661 University 12 Avenue, Toronto, ON M5G 0A3 Canada 13 *Corresponding author at: Biology Department, Pace University, 1 Pace Plaza, New 14 York, NY 10038; email: [email protected] 15 16 Abstract 17 The phenotypic, geographic, and species diversity of cichlid fishes have made them a 18 group of great interest for studying evolutionary processes. Here we present a targeted- 19 exon next-generation sequencing approach for investigating the evolutionary 20 relationships of cichlid fishes (Cichlidae), with a particular focus on the Neotropical 21 subfamily Cichlinae using a set of 923 primarily single-copy exons designed through 22 mining of the Nile tilapia (Oreochromis niloticus) genome. Sequence capture and 23 assembly were robust, leading to a complete dataset of 415 exons for 139 species (147 1 bioRxiv preprint doi: https://doi.org/10.1101/133512; this version posted May 2, 2017. -

Apistogramma Erythrura Sp. N. – a New Geophagine Dwarf Cichlid (Teleostei: Perciformes: Cichlidae) from the Río Mamoré Drainage in Bolivia
Vertebrate Zoology 58 (2) 2008 197 197 – 206 © Museum für Tierkunde Dresden, ISSN 1864-5755, 26.11.2008 Apistogramma erythrura sp. n. – a new geophagine dwarf cichlid (Teleostei: Perciformes: Cichlidae) from the río Mamoré drainage in Bolivia WOLFGANG STAECK 1 & INGO SCHINDLER 2 1 Auf dem Grat 41a, D-14195 Berlin 2 Warthestr. 53a, D-12051 Berlin > [email protected] Received on May 15, 2008, accepted on May 28, 2008. Published online at www.vertebrate-zoology.de on November 21, 2008. > Abstract Apistogramma erythrura sp.n. is described from the drainage of the lower río Mamoré in the province of Beni in Bolivia. It is most similar to Apistogramma trifasciata, but differs from this species by the complete absence of any abdominal markings below the lateral band. It can be distinguished from all the other described Apistogramma species by the following combination of characters: only two postlacrymal infraorbital lateralis canal pores, fi ve dentary lateralis canal pores, a broad lateral band with even borders that widens posteriorly to a height of at least two and a half scales and extends into the caudal fi n without a caudal spot. Adult males usually with a partly or even completely bright red colouration of their rounded caudal fi n, extremely produced anterior dorsal-fi n lappets and prolonged pelvic-fi n fi laments reaching the posterior end of the anal- fi n base. > Kurzfassung Apistogramma erythrura sp. n. wird aus dem Fluss-System des unteren río Mamoré aus der Provinz Beni in Bolivien beschrieben. Die neue Art ist Apistogramma trifasciata am ähnlichsten, unterscheidet sich von jener Spezies jedoch durch das völlige Fehlen dunkler Zeichnungselemente unterhalb des Lateralbandes.