Chimera Proposal
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Human Chimera: Legal Problems Arising from Individuals with Multiple Types of Dna
Seton Hall University eRepository @ Seton Hall Law School Student Scholarship Seton Hall Law 5-2-2014 The umH an Chimera: Legal Problems Arising From Individuals with Multiple Types of DNA Robert Russell Granzen Follow this and additional works at: https://scholarship.shu.edu/student_scholarship Recommended Citation Granzen, Robert Russell, "The umH an Chimera: Legal Problems Arising From Individuals with Multiple Types of DNA" (2014). Law School Student Scholarship. 485. https://scholarship.shu.edu/student_scholarship/485 THE HUMAN CHIMERA: LEGAL PROBLEMS ARISING FROM INDIVIDUALS WITH MULTIPLE TYPES OF DNA Robert Granzen INTRODUCTION Science continually changes, and with it our understanding of the human body. While some scientific developments are limited in scope, others have widespread effects. Scientists have just recently begun understanding the range of effects chimerism in humans can have. Chimerism, originally associated with hermaphrodites having both male and female sexual organs, is much more common than originally thought. As chimerism becomes more common, so do individuals with separate and distinct deoxyribonucleic acid (DNA) strands in their bodies. Most individuals are unaware of their chimeric genetic code and most will likely never know. Because of the inherent difficulty of testing for chimerism, many problems are presented to legal system. Part I of this article will begin with the history of human chimeras. The section will then describe the ways in which chimeras are formed. The section will discuss the most common form of chimerism in humans--fetal cell microchimerism (FMC). FMC occurs when cells are transferred from baby to mother or mother to baby via the umbilical cord.1 Studies have shown that mothers may keep cells from their children for years after giving birth.2 Moreover, cells can be exchanged between twins while inside the uterus.3 Secondly, the section will describe the process of embryo fusion, which can cause tetragametic chimerism. -

Patentability of Human-Animal Chimeras Ryan Hagglund
Santa Clara High Technology Law Journal Volume 25 | Issue 1 Article 4 2008 Patentability of Human-Animal Chimeras Ryan Hagglund Follow this and additional works at: http://digitalcommons.law.scu.edu/chtlj Part of the Law Commons Recommended Citation Ryan Hagglund, Patentability of Human-Animal Chimeras, 25 Santa Clara High Tech. L.J. 51 (2012). Available at: http://digitalcommons.law.scu.edu/chtlj/vol25/iss1/4 This Article is brought to you for free and open access by the Journals at Santa Clara Law Digital Commons. It has been accepted for inclusion in Santa Clara High Technology Law Journal by an authorized administrator of Santa Clara Law Digital Commons. For more information, please contact [email protected]. PATENTABILITY OF HUMAN-ANIMAL CHIMERAS Ryan Hagglundt Abstract The chimera was a mythological creature with a lion 's head, goat's body, and serpent's tail. Because of recent advances in biotechnology, such permutations on species are no longer the stuff of myth and legend The term "chimera " has come to describe a class of genetically engineered creatures composed of some cells from one species, which thus contain genetic material derived entirely from that species, and some cells from another species, containing only genetic material from that species. Scientists have created a goat- sheep chimera or "geep" which exhibits physical characteristicsof both animals. Likewise, scientists have also used the tools of modern molecular biology to create human-animal chimeras containing both human and animal cells. While none of the human-animal chimeras hitherto created have exhibited significant human characteristics,the synthesis of human-animal chimeras raises significant ethical concerns. -

My Attempt to Patent a Human-Animal Chimera
No 27 - avril-mai 2006 My attempt to patent a human-animal chimera In 1997, with the support of the social critic Jeremy Rifkin of the Foundation on Economic Trends in Washington, D.C., Professor Stuart A. Newman undertook to apply for a patent on embryos and full- term creatures containing human along with nonhuman cells – so-called “chimeras” that he had no intention of producing... Stuart A. Newman* In 1997, with the support of the social critic Jeremy Rifkin of the Foundation on Economic Trends in Washington, D.C., I undertook to apply for a patent on embryos and full-term creatures containing human along with nonhuman cells – so-called “chimeras”. The public had only just learned (to widespread surprise) about the cloning of Dolly the sheep and the announcement of the production of versatile and controversial embryonic stem (ES) cells from human embryos was still a year in the future. In those innocent days less than a decade ago the prospect of fabricating creatures that were part-human and part-beast would have appeared to most people like something out of Greek mythology or the pages of a fantasy novel. Indeed, it had been a century since the British writer H.G. Wells had published “The Island of Dr. Moreau”, the story of a visionary scientist whose well-motivated experiments along these lines led to death and destruction. Still, in the last years of the 20th century, such quasi-humans were unknown outside of works of fiction. Nonetheless, as a developmental biologist whose work requires close tracking of the scientific literature, I knew that such things were feasible. -

An Interesting Case of Chimera: Inconclusive BRCA Analysis Due To
Case Report iMedPub Journals 2018 www.imedpub.com Journal of Genomics & Gene Study Vol.1 No.1:3 An Interesting Case of Chimera: Inconclusive Bowers AW*, Monteverde A, BRCA Analysis due to Two Distinct Genetic Keckeisen G and Kapenhas E Profiles in a Single Patient Ellen Hermanson Breast Center, Southampton, New York, USA Abstract *Corresponding author: Anne Wills Bowers Background: Our case study proposes an explanation for the inconclusive BRCA testing we received on one of our patients. The BRCA analysis was inconclusive [email protected] due to the specimen being chimeric, or having two distinct genetic profiles. We propose the Vanishing Twin theory as the most probable explanation. We have Ellen Hermanson Breast Center, found no other reported cases of chimera linked to Vanishing Twin Syndrome in Southampton, New York, USA. the literature. Methods: In this case report, we have described the presentation and operative Tel: +1 631-726-8400 course of a single patient of our Breast Center in 2017 to help define the setting of our unique BRCA analysis result. Findings: We have also done an extensive literature search in an attempt to Citation: Bowers AW, Monteverde A, understand the implications of the BRCA analysis result in a chimeric patient. Keckeisen G, Kapenhas E (2018) An However, our search was not satisfied by any direct evidence. We can only Interesting Case of Chimera: Inconclusive conclude that the Vanishing Twin theory is the most likely explanation of chimera BRCA Analysis due to Two Distinct Genetic in this single patient. Profiles in a Single Patient. J Genom Gene Study Vol.1 No.1:3 Conclusions: We were unable to find any direct explanation for our results but we do propose this as an interesting case, as chimera is rare. -
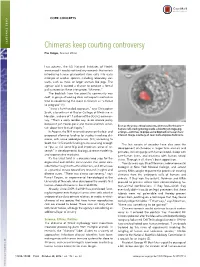
Chimeras Keep Courting Controversy Elie Dolgin, Science Writer
CORE CONCEPTS CORE CONCEPTS Chimeras keep courting controversy Elie Dolgin, Science Writer Last autumn, the US National Institutes of Health announced it would not fund any research that entails introducing human pluripotent stem cells into early embryos of another species, including laboratory stal- warts, such as mice, or larger animals like pigs. The agency said it wanted a chance to conduct a formal policy review on these interspecies “chimeras.” The backlash from the scientific community was swift. A group of leading stem cell experts wasted no time in condemning the move in Science as “a threat to progress” (1). “It was a ham-handed approach,” says Christopher Scott, a bioethicist at Baylor College of Medicine in Houston, and one of 11 authors of the Science commen- tary. “That’s a really terrible way to do science policy because it just creates panic and makes scientists uncer- — ” Even as they raise ethical concerns, chimeras like this one tain about their lines of inquiry. human cells (red) growing inside a blastocyst-stage pig In August, the NIH reversed course on the ban and embryo—continue to pique some biomedical researchers’ proposed allowing funding for studies involving chi- interest. Image courtesy of Juan Carlos Izpisua ´ Belmonte. meras, with some added provisos. Still, according to Scott, the 10.5-month funding hiatus was long enough The last couple of decades have also seen the “ to put at risk some big and important areas of re- development of chimeras in larger farm animals and ” search in developmental biology, disease modeling, primates, including pigs with human blood, sheep with and regenerative medicine. -

Human-Nonhuman Chimeras: a Regulatory Proposal on the Blurring of Species Lines Nicole E
Boston College Law Review Volume 45 Article 3 Issue 3 Number 3 5-1-2004 Human-Nonhuman Chimeras: A Regulatory Proposal on the Blurring of Species Lines Nicole E. Kopinski Follow this and additional works at: http://lawdigitalcommons.bc.edu/bclr Part of the Science and Technology Law Commons Recommended Citation Nicole E. Kopinski, Human-Nonhuman Chimeras: A Regulatory Proposal on the Blurring of Species Lines, 45 B.C.L. Rev. 619 (2004), http://lawdigitalcommons.bc.edu/bclr/vol45/iss3/3 This Notes is brought to you for free and open access by the Law Journals at Digital Commons @ Boston College Law School. It has been accepted for inclusion in Boston College Law Review by an authorized editor of Digital Commons @ Boston College Law School. For more information, please contact [email protected]. HUMAN-NONHUMAN CHIMERAS: A REGULATORY PROPOSAL ON THE BLURRING OF SPECIES LINES Abstract: The chimera of modern biotechnology is defined broadly as a single organism composed of a mixture of materials from two or more organisms possessing distinct genetic backgrounds. Unlike the United States, which does not regulate chimeras directly, Canada has responded to the unregulated pursuit of chimera technology by banning certain chim- eras as part of comprehensive legislation designed to regulate human reproductive technologies. In 2001, the Canadian Parliament passed the Assisted Human Reproduction Act despite criticism urging greater legis- lative justification for the Act's provisions and modification to its statutory definitions, Because current regulatory mechanisms in the United States, including patent law and administrative oversight, fail to regulate chimera technology, the United States should enact new legislation, using Canada's legislation as a model, to prohibit embryonic chimeras and to regulate other human-nonhuman combinations. -

3257.Full.Pdf
(CANCER RESEARCH 51. 3257-3260. June 15. 1991] Strain Specific Sensitivity to Diethylnitrosamine-induced Carcinogenesis Is Maintained in Hepatocytes of C3H/HeN^C57BL/6N Chimeric Mice1 Gang-Hong Lee,2 Kimie Nomura, Hiroaki Kanda, Moriaki Kusakabe, Atsushi Voshiki, Teruyo Sakakura, and Tomoyuki Kitagawa Department of Pathology, Cancer Institute, Toshima-ku, Tokyo 171)/ii-H. L, K. N., H. A'., 7".A'./, anil Laboratory of Cell Biology, Tsukuba Life Science Center, The Institute of Physical and Chemical Research (RIKE.\), Ibaraki 305 [M. A".,A. Y., T. S.J, Japan ABSTRACT derived neoplasms (21 of 23) in their results actually reflected numbers or rather only differences in growth rate of initiated The C3H/HeN (C3H) and C57BL/6N (C57) mouse strains are known, or preneoplastic lesions of C3H and C57 types in the chimeric respectively, for their high and low susceptibility to both spontaneous mouse liver. and chemically induced hepatocarcinogenesis. The present study was aimed at elucidating whether this difference is dependent on intrinsic It has been shown in rodent hepatocarcinogenesis that the features of the target hepatocytes or the in vivo milieu and associated effects of initiation and promotion can be reliably and quanti growth promoting factors to which the cells are exposed. C3H«-»C57tatively estimated by scoring altered cell lesions, which are the chimeric mice were produced and given injections of diethylnitrosamine proliferated progenies of carcinogen-initiated cells (6-8). For (20 ¿ig/gbodyweight) at the age of 15 days. The animals were sacrificed example, previous studies have shown that both the number 6 or 9 months thereafter, and the numbers and sizes of altered cell lesions and size of microscopically detectable lesions induced by DEN were scored. -

An Interspecies Barrier to Tetraploid Complementation and Chimera Formation
www.nature.com/scientificreports OPEN An interspecies barrier to tetraploid complementation and chimera formation Received: 19 February 2018 Tomoyuki Yamaguchi 1, Hideyuki Sato1, Toshihiro Kobayashi1,5, Megumi Kato-itoh1, Accepted: 3 October 2018 Teppei Goto 2, Hiromasa Hara2, Naoaki Mizuno1, Ayaka Yanagida1,6, Ayumi Umino1, Published: xx xx xxxx Sanae Hamanaka1, Fabian Suchy4, Hideki Masaki1, Yasunori Ota3, Masumi Hirabayashi2 & Hiromitsu Nakauchi1,4 To study development of the conceptus in xenogeneic environments, we assessed interspecies chimera formation as well as tetraploid complementation between mouse and rat. Overall contribution of donor PSC-derived cells was lower in interspecies chimeras than in intraspecies chimeras, and high donor chimerism was associated with anomalies or embryonic death. Organ to organ variation in donor chimerism was greater in interspecies chimeras than in intraspecies chimeras, suggesting species- specifc afnity diferences among interacting molecules necessary for organogenesis. In interspecies tetraploid complementation, embryo development was near normal until the stage of placental formation, after which no embryos survived. Advances in chimera and stem cell technology has provided intriguing tools with many basic and translational applications. Since chimeras have at least two cell populations with diferent genetic backgrounds, they can be used to compare cell potency and function, or probe signaling pathways during development. Generation of chi- meras using PSCs is well-established for intraspecies applications1–7, however is more difcult in an interspecies setting. One exciting interspecies application exploits mouse-rat chimerism to generate PSC-derived organs by blastocyst complementation8. Injection of rat induced PSCs (iPSCs) into Pdx1−/− mouse blastocysts successfully generated a rat pancreas in a mouse, demonstrating that PSCs can contribute to xenogenic embryo development with postnatal survival. -

Chimeric Brains Consisted of Primatologists and Other Scientists, Ethicists, and Lawyers
Chimeras Definition Ethical Issues Links Suggested Reading End Notes Definition: What are Chimeras? How and why are they produced? Chimeras are animals composed of cells that originate from two (or more) different species. In the research lab, chimeras are created by introducing cells from one species into the developing embryo or fetus of another. (The name chimera comes from Greek mythology and describes a creature with the head of a lion, the body of a goat, and the tail of a serpent). The first chimeras helped scientists understand questions about developmental biology. A sheep-goat chimera, created in 1984, had the head of a goat and the woolly coat of a sheep.i Chicken-quail chimeras have also been successfully developed.ii,iii Sheep-Goat Chimera Courtesy of Dr. Gary B. Anderson -University of California, Davis Now, researchers are developing human-animal chimeras to study disease processes, test new drugs, and develop organs for future transplant patients. The chimeras are produced by introducing human stem cells into developing animal embryos. The following are some of the major projects utilizing human- animal chimeras underway in the U.S and internationally: Sheep-to-humans organ transplant project: At the University of Nevada at Reno, researchers have added human stem cells to sheep fetuses to create chimeras that they hope will someday serve as a reliable source of organs for liver transplant patients. Some sheep now have livers with up to 80% human cells that produce the compounds normally made by human livers. (The cells are not all in distinct liver lobes but spread throughout the tissue. -

Human-Animal Chimeras for Autologous Organ Transplantation: Technological Advances and Future Perspectives
576 Review Article Page 1 of 8 Human-animal chimeras for autologous organ transplantation: technological advances and future perspectives Yingfei Lu1, Yu Zhou1,2, Rong Ju2, Jianquan Chen1,2 1Central Laboratory, Translational Medicine Research Center, 2Department of Obstetrics and Gynecology, The Affiliated Jiangning Hospital with Nanjing Medical University, Nanjing 211100, China Contributions: (I) Conception and design: All authors; (II) Administrative support: None; (III) Provision of study materials or patients: None; (IV) Collection and assembly of data: None; (V) Data analysis and interpretation: None; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors. Correspondence to: Jianquan Chen. Central Laboratory, Translational Medicine Research Center, The Affiliated Jiangning Hospital with Nanjing Medical University, Nanjing 211100, China. Email: [email protected]. Abstract: Organ transplantation is the most promising curation for end-stage organ disease. However, the donor organ shortage has become a global problem that has limited the development of organ transplantation. Human-animal chimeras provide the ability to produce human organs in other species using autologous stem cells [e.g., induced pluripotent stem cells (iPSCs) or adult stem cells], which would be patient-specific and immune-matched for transplantation. Due to the potential application prospect of interspecies chimeras in basic and translational research, this technology has attracted much interest. This review focuses primarily on technological advances, including options of donor stem cell types and gene editing in donor cells and host animals, in addition to perspectives on human-animal chimeras in clinical and basic research. Keywords: Autologous stem cells; embryo complementation; gene editing; human-animal chimeras; organ transplantation Submitted Jul 17, 2019. -

Human-Animal Chimeras
Issue Brief June 2021 | No. IF21F01 Human-Animal Chimeras: Unethical and Unnecessary by Mary Szoch, M.Ed. Key Points n the United States, the ethical considerations regarding Human-animal chimeras are human-animal chimeras and human embryo research are creatures that are part human and part animal. Since 1979, intertwined. Understanding the regulations on human- there has been a restriction, Ianimal chimeras the United States ought to have requires a known as the 14-Day Rule, on developing this type of review of the United States’ policies on human embryo creature past 14 days gestation research, an overview of what human-animal chimeras are, an in vitro. analysis of the purported benefits of human-animal chimera In 2016, after scientists gained the ability to develop an research, and a synopsis of the ethical questions posed by embryo in vitro beyond nine creating these creatures. days, the National Institutes of Health revisited the 14-Day Rule, but under the pro-life Trump administration, the Rule The serious ethical concerns about the creation of human- was kept in place. animal chimeras and the lack of any beneficial scientific After a partnership with China advancements from chimera experiments make it clear that created the first human- primate chimera, the NIH, human-animal chimeras are unethical and unnecessary. under the Biden administration, opted to once again review the 14-Day Rule. Senators recently voted along party lines against banning the creation of human- animal chimeras through legislation. This report can be read online at frc.org/chimeras 801 G. St. NW Washington, D.C. -

Analysis of Homozygous Mutant Chimeric Mice: Deletion Of
Proc. Natl. Acad. Sci. USA Vol. 90, pp. 2551-2555, March 1993 Genetics Analysis of homozygous mutant chimeric mice: Deletion of the immunoglobulin heavy-chain joining region blocks B-cell development and antibody production (B-cell deficiency/immunoglobulin gene rearrangement/gene targeting/embryonic stem cells) AYA JAKOBOVITS*, GERMAN J. VERGARA, JACQUELINE L. KENNEDY, JOANNA F. HALES, RYAN P. MCGUINNESS, DENISE E. CASENTINI-BOROCZ, DANIEL G. BRENNER, AND GILLIS R. OTTEN Cell Genesys, Inc., 322 Lakeside Drive, Foster City, CA 94404 Communicated by Ronald W. Davis, November 18, 1992 ABSTRACT Using a recently described method for effi- MATERIALS AND METHODS ciently deriving homozygous targeted alleles in embryonic stem cells, we produced chimeric mice whose tissues were derived Targeting-Vector Construction. Plasmid pUC18-JH, con- partially from embryonic stem cells bearing homozygous de- taining a 6.1-kb EcoRI genomic fragment of the mouse Ig letion of the mouse immunoglobulin heavy-chain joining (JH) heavy-chain (Igh) locus (8), was digested with Xho I and Nae region. Characterization of these chimeric mice indicated that I to delete the 2296-bp fragment containing the entire JH of development at region and the DQ52 gene segment, which was replaced by a homozygous JH deletion leads to arrest B-cell 1150-bp Xho I-BamHI fragment of pMClNeopolA (9, 10) to an early stage, resulting in a total lack ofperipheral B cells and form the targeting vector pmHAJ. The unique pUC-derived serum IgM. These results were confirmed in mice containing Nde I site was used for linearization. the homozygous JH deletion in their germ line.