The V2 Receptor Antagonists
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Science of Medicine. the Art of Healing. Going Lecture Free for Genz
The science of medicine. The art of healing. Going Lecture Free for GenZ Brenda J.B. Roman, M.D. Associate Dean for Medical Education Irina Overman, M.D. Director of Foundations Curriculum Mary Jo Trout, Pharm.D. Director of Therapeutics Curriculum Image obtained from: https://visual.ly/community/infographic/other/generation-z Gen Z Learning Preferences ▪ “Hands on” learning opportunities ▪ Application to “real Life” ▪ Desire community engagement ▪ “Observers” first ▪ Value independent learning ▪ Differs from Millennials (teamwork-oriented approach) ▪ View peers and instructors as valuable resources No attendance problems What we did… Science of Learning Research… What doesn’t work Cognitive illusions ▪ Ineffective strategies that produce massive overconfidence Popular ineffective strategies ▪ Passive repetitive reading ▪ Highlighting and underlining ▪ Summarization ▪ Keyword mnemonics ▪ Imagery for text Science of Learning Research… What does work ▪ Interleaved practice ▪ Elaborative interrogation ▪ Self-explanation ▪ Distributed practice ▪ Practice testing ▪ Retrieval-based learning Foster a Growth Mindset ➢ Willingness to learn ➢ Get comfortable with from reading less than full mastery of material before the session Peer Multiple-Choice Instruction Questions Team-Based WrightQ Learning WrightCurriculum DISTRIBUTED PRACTICE INTERLEAVING READING Peer Instruction Students answer problem sets individually with ARS, then peer instruct and re-answer individually Retrieval-based learning Elaborative interrogation Self-explanation Practice -

AHFS Pharmacologic-Therapeutic Classification System
AHFS Pharmacologic-Therapeutic Classification System Abacavir 48:24 - Mucolytic Agents - 382638 8:18.08.20 - HIV Nucleoside and Nucleotide Reverse Acitretin 84:92 - Skin and Mucous Membrane Agents, Abaloparatide 68:24.08 - Parathyroid Agents - 317036 Aclidinium Abatacept 12:08.08 - Antimuscarinics/Antispasmodics - 313022 92:36 - Disease-modifying Antirheumatic Drugs - Acrivastine 92:20 - Immunomodulatory Agents - 306003 4:08 - Second Generation Antihistamines - 394040 Abciximab 48:04.08 - Second Generation Antihistamines - 394040 20:12.18 - Platelet-aggregation Inhibitors - 395014 Acyclovir Abemaciclib 8:18.32 - Nucleosides and Nucleotides - 381045 10:00 - Antineoplastic Agents - 317058 84:04.06 - Antivirals - 381036 Abiraterone Adalimumab; -adaz 10:00 - Antineoplastic Agents - 311027 92:36 - Disease-modifying Antirheumatic Drugs - AbobotulinumtoxinA 56:92 - GI Drugs, Miscellaneous - 302046 92:20 - Immunomodulatory Agents - 302046 92:92 - Other Miscellaneous Therapeutic Agents - 12:20.92 - Skeletal Muscle Relaxants, Miscellaneous - Adapalene 84:92 - Skin and Mucous Membrane Agents, Acalabrutinib 10:00 - Antineoplastic Agents - 317059 Adefovir Acamprosate 8:18.32 - Nucleosides and Nucleotides - 302036 28:92 - Central Nervous System Agents, Adenosine 24:04.04.24 - Class IV Antiarrhythmics - 304010 Acarbose Adenovirus Vaccine Live Oral 68:20.02 - alpha-Glucosidase Inhibitors - 396015 80:12 - Vaccines - 315016 Acebutolol Ado-Trastuzumab 24:24 - beta-Adrenergic Blocking Agents - 387003 10:00 - Antineoplastic Agents - 313041 12:16.08.08 - Selective -

Conivaptan (Vaprisol )
January 3, 2007 Conivaptan (Vaprisol ®) DESCRIPTION WHAT YOU MAY NOT BE TOLD Conivaptan (Vaprisol ®) is an inhibitor of arginine Conivaptan is currently approved for euvolemic vasopressin that prevents its binding to V1a and V2 hyponatremia, but the only peer-reviewed published receptors located within the vasculature and renal data is in acute exacerbations of heart failure. It is tubules, respectively. Conivaptan was approved likely that the drug manufacturer will pursue this December 30, 2005, and is a first-in-class agent indication in the future. Conivaptan is also a potent approved for euvolemic hyponatremia. Conivaptan is inhibitor of CYP 3A4 and, as an oral formulation, available in 20 mg/5mL glass ampules. increases the area under the curve of midazolam 2- to 3-fold, simvastatin 3-fold, and amlodipine 2-fold. This WHAT THE PHARMACIST SHOULD KNOW level of interaction is what prevented the oral Euvolemic hyponatremia is most commonly associated formulation from being pursued for FDA approval. with the syndrome of inappropriate antidiuretic hormone secretion (SIADH). Causes of this syndrome WHAT THE PATIENT SHOULD KNOW include pulmonary disease (e.g., pneumonia, Conivaptan is used to treat euvolemic hyponatremia, tuberculosis, pleural effusion) and CNS disease (e.g., manifested when a patient has too little sodium in the tumor, subarachnoid hemorrhage, meningitis). SIADH bloodstream but a normal amount of fluid in the body. also occurs with malignancies (e.g., small cell Conivaptan should not be used at this time for heart carcinoma of the lung) and can be caused by drugs failure. Conivaptan has relatively few adverse effects (e.g., chlorpropamide, carbamazepine, narcotic and is well tolerated. -

Subject: Samsca (Tolvaptan) Original Effective Date: 07/27/15
Subject: Samsca (tolvaptan) Original Effective Date: 07/27/15 Policy Number: MCP-252 Revision Date(s): Review Date(s): 12/15/2016; 6/22/2017 DISCLAIMER This Medical Policy is intended to facilitate the Utilization Management process. It expresses Molina's determination as to whether certain services or supplies are medically necessary, experimental, investigational, or cosmetic for purposes of determining appropriateness of payment. The conclusion that a particular service or supply is medically necessary does not constitute a representation or warranty that this service or supply is covered (i.e., will be paid for by Molina) for a particular member. The member's benefit plan determines coverage. Each benefit plan defines which services are covered, which are excluded, and which are subject to dollar caps or other limits. Members and their providers will need to consult the member's benefit plan to determine if there are any exclusion(s) or other benefit limitations applicable to this service or supply. If there is a discrepancy between this policy and a member's plan of benefits, the benefits plan will govern. In addition, coverage may be mandated by applicable legal requirements of a State, the Federal government or CMS for Medicare and Medicaid members. CMS's Coverage Database can be found on the CMS website. The coverage directive(s) and criteria from an existing National Coverage Determination (NCD) or Local Coverage Determination (LCD) will supersede the contents of this Molina Clinical Policy (MCP) document and provide the directive for all Medicare members. SUMMARY OF EVIDENCE/POSITION This policy addresses the coverage of Samsca (tolvaptan) for the treatment of clinically significant hypervolemic and euvolemic hyponatremia when appropriate criteria are met. -

Diuretics in Clinical Practice. Part I: Mechanisms of Action, Pharmacological Effects and 1
Review Diuretics in clinical practice. Part I: mechanisms of action, pharmacological effects and 1. Introduction clinical indications of diuretic 2. Principles of diuretic action and classification of diuretic compounds compounds † 3. Carbonic anhydrase inhibitors Pantelis A Sarafidis , Panagiotis I Georgianos & Anastasios N Lasaridis Aristotle University of Thessaloniki, AHEPA Hospital, Section of Nephrology and Hypertension, 4. Osmotic diuretics 1st Department of Medicine, Thessaloniki, St. Kiriakidi 1, 54636, Thessaloniki, Greece 5. Loop diuretics 6. Thiazides and thiazide-related Importance of the field: Diuretics are among the most important drugs of our diuretics therapeutic armamentarium and have been broadly used for > 50 years, 7. Potassium-retaining diuretics providing important help towards the treatment of several diseases. Although all diuretics act primarily by impairing sodium reabsorption in the renal 8. Conclusion tubules, they differ in their mechanism and site of action and, therefore, in 9. Expert opinion their specific pharmacological properties and clinical indications. Loop diure- tics are mainly used for oedematous disorders (i.e., cardiac failure, nephrotic syndrome) and for blood pressure and volume control in renal disease; thiazides and related agents are among the most prescribed drugs for hypertension treatment; aldosterone-blockers are traditionally used for primary or secondary aldosteronism; and other diuretic classes have more specific indications. Areas covered in this review: This article discusses the mechanisms of action, pharmacological effects and clinical indications of the various diuretic classes For personal use only. used in everyday clinical practice, with emphasis on recent knowledge sug- gesting beneficial effects of certain diuretics on clinical conditions distinct from the traditional indications of these drugs (i.e., heart protection for aldosterone blockers). -

Mineralocorticoid Receptor Antagonists for Heart Failure with Reduced Ejection Fraction: Integrating Evidence Into Clinical Practice
European Heart Journal Advance Access published August 31, 2012 European Heart Journal REVIEW doi:10.1093/eurheartj/ehs257 Novel Therapeutic Concepts Mineralocorticoid receptor antagonists for heart failure with reduced ejection fraction: integrating evidence into clinical practice Faiez Zannad1*, Wendy Gattis Stough2,PatrickRossignol1, Johann Bauersachs3, John J.V. McMurray4,KarlSwedberg5,AllanD.Struthers6, Adriaan A. Voors7, Luis M. Ruilope8,GeorgeL.Bakris9, Christopher M. O’Connor10, Mihai Gheorghiade11, Downloaded from Robert J. Mentz10, Alain Cohen-Solal12,AldoP.Maggioni13, Farzin Beygui14, Gerasimos S. Filippatos15,ZiadA.Massy16, Atul Pathak17, Ileana L. Pin˜a18, Hani N. Sabbah19, Domenic A. Sica20, Luigi Tavazzi21, and Bertram Pitt22 http://eurheartj.oxfordjournals.org/ 1INSERM, Centre d’Investigation Clinique 9501 and Unite´ 961, Centre Hospitalier Universitaire, and the Department of Cardiology, Nancy University, Universite´ de Lorraine, Nancy, France; 2Campbell University College of Pharmacy and Health Sciences, Buies Creek, NC, USA; 3Department of Cardiology and Angiology, Hannover Medical School, Hannover, Germany; 4Western Infirmary and the British Heart Foundation, Glasgow Cardiovascular Research Centre, University of Glasgow, Glasgow, UK; 5Department of Molecular and Clinical Medicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden; 6University of Dundee, Ninewells Hospital and Medical School, Dundee, UK; 7University Medical Center Groningen, University of Groningen, Groningen, The Netherlands; 8Hypertension -
![Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set](https://docslib.b-cdn.net/cover/8870/ehealth-dsi-ehdsi-v2-2-2-or-ehealth-dsi-master-value-set-1028870.webp)
Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set
MTC eHealth DSI [eHDSI v2.2.2-OR] eHealth DSI – Master Value Set Catalogue Responsible : eHDSI Solution Provider PublishDate : Wed Nov 08 16:16:10 CET 2017 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 1 of 490 MTC Table of Contents epSOSActiveIngredient 4 epSOSAdministrativeGender 148 epSOSAdverseEventType 149 epSOSAllergenNoDrugs 150 epSOSBloodGroup 155 epSOSBloodPressure 156 epSOSCodeNoMedication 157 epSOSCodeProb 158 epSOSConfidentiality 159 epSOSCountry 160 epSOSDisplayLabel 167 epSOSDocumentCode 170 epSOSDoseForm 171 epSOSHealthcareProfessionalRoles 184 epSOSIllnessesandDisorders 186 epSOSLanguage 448 epSOSMedicalDevices 458 epSOSNullFavor 461 epSOSPackage 462 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 2 of 490 MTC epSOSPersonalRelationship 464 epSOSPregnancyInformation 466 epSOSProcedures 467 epSOSReactionAllergy 470 epSOSResolutionOutcome 472 epSOSRoleClass 473 epSOSRouteofAdministration 474 epSOSSections 477 epSOSSeverity 478 epSOSSocialHistory 479 epSOSStatusCode 480 epSOSSubstitutionCode 481 epSOSTelecomAddress 482 epSOSTimingEvent 483 epSOSUnits 484 epSOSUnknownInformation 487 epSOSVaccine 488 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 3 of 490 MTC epSOSActiveIngredient epSOSActiveIngredient Value Set ID 1.3.6.1.4.1.12559.11.10.1.3.1.42.24 TRANSLATIONS Code System ID Code System Version Concept Code Description (FSN) 2.16.840.1.113883.6.73 2017-01 A ALIMENTARY TRACT AND METABOLISM 2.16.840.1.113883.6.73 2017-01 -

Hypernatremia: 2 Months Back When He Had Progressive Decrease in Vision (Right > Left)
Correspondence to increased intrathoracic pressure and central radiologist regarding vascular injury when there is venous pressure, decreased venous return from the refractory hypotension so that necessary steps can be brain and increased ICP[4] taken to identify and treat iatrogenic complications that • Patients with aneurysmal SAH have disturbed might compound the pre‑existing morbidity. autoregulation of cerebral blood flow. Hence, intraoperative hypotension of any cause has an REFERENCES adverse effect on the outcome of SAH[5,6] • Patients with poor grade SAH (Fischer III/IV) and 1. Young WL, Pile-Spellman J. Anesthetic considerations for interventional Neuroradiolgy (Review). Anesthesiology angiographic evidence of vasospasm which our 1994;80:427-56. patient had, are at risk for cerebral hypoperfusion 2. Varma MK, Price K, Jayakrishnan V, Manickam B, Kessell G. because of impaired cerebral autoregulation and Anaesthetic considerations for interventional neuroradiology. thus intraoperative hypotension and cerebral Br J Anaesth 2007;99:75-85. [6,7] 3. Kent KC, Moscucci M, Mansour KA, Dimattia S, Gallagher S, hypoperfusion compounds the poor outcome Kuntz R, et al. Retroperitoneal hematoma after cardiac • Blood loss leading to severe anaemia (Hb 3.8 g%) catheterization: Prevalence, risk factors, and optimal during the procedure might have lead to decreased management. J Vasc Surg 1994;20:905-10. oxygen‑carrying capacity of blood and further 4. De Laet I, Citerio G, Malbrain ML. The influence of exacerbate the hypoxic injury to brain. intraabdominal hypertension on the central nervous system: Current insights and clinical recommendations, is it all in the Thus a combination of age, poor grade SAH, diffuse head? Acta Clin Belg Suppl 2007;1:89-97. -

Pharmaceutical Appendix to the Tariff Schedule 2
Harmonized Tariff Schedule of the United States (2007) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2007) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. ABACAVIR 136470-78-5 ACIDUM LIDADRONICUM 63132-38-7 ABAFUNGIN 129639-79-8 ACIDUM SALCAPROZICUM 183990-46-7 ABAMECTIN 65195-55-3 ACIDUM SALCLOBUZICUM 387825-03-8 ABANOQUIL 90402-40-7 ACIFRAN 72420-38-3 ABAPERIDONUM 183849-43-6 ACIPIMOX 51037-30-0 ABARELIX 183552-38-7 ACITAZANOLAST 114607-46-4 ABATACEPTUM 332348-12-6 ACITEMATE 101197-99-3 ABCIXIMAB 143653-53-6 ACITRETIN 55079-83-9 ABECARNIL 111841-85-1 ACIVICIN 42228-92-2 ABETIMUSUM 167362-48-3 ACLANTATE 39633-62-0 ABIRATERONE 154229-19-3 ACLARUBICIN 57576-44-0 ABITESARTAN 137882-98-5 ACLATONIUM NAPADISILATE 55077-30-0 ABLUKAST 96566-25-5 ACODAZOLE 79152-85-5 ABRINEURINUM 178535-93-8 ACOLBIFENUM 182167-02-8 ABUNIDAZOLE 91017-58-2 ACONIAZIDE 13410-86-1 ACADESINE 2627-69-2 ACOTIAMIDUM 185106-16-5 ACAMPROSATE 77337-76-9 -

The Syndrome of Inappropriate Antidiuretic Hormone: Current and Future Management Options
European Journal of Endocrinology (2010) 162 S13–S18 ISSN 0804-4643 The syndrome of inappropriate antidiuretic hormone: current and future management options Mark Sherlock and Chris J Thompson1 Centre for Endocrinology, Diabetes and Metabolism, School of Clinical and Experimental Medicine, University of Birmingham, Birmingham, B15 2TT, UK and 1Academic Department of Diabetes and Endocrinology, Beaumont Hospital and RCSI Medical School, Beaumont Road, Dublin 9, Ireland (Correspondence should be addressed to C J Thompson; Email: [email protected]) Abstract Hyponatraemia is the commonest electrolyte abnormality, and syndrome of inappropriate antidiuretic hormone (SIADH) is the most frequent underlying pathophysiology. Hyponatraemia is associated with significant morbidity and mortality, and as such appropriate treatment is essential. Treatment options for SIADH include fluid restriction, demeclocycline, urea, frusemide and saline infusion, all of which have their limitations. The introduction of the vasopressin-2 receptor antagonists has allowed clinicians to specifically target the underlying pathophysiology of SIADH. Initial studies have shown good efficacy and safety profiles in the treatment of mild to moderate hyponatraemia. However, studies assessing the efficacy and safety of these agents in acute severe symptomatic hyponatraemia are awaited. Furthermore, the cost of these agents at present may limit their use. European Journal of Endocrinology 162 S13–S18 Introduction diagnosis of SIADH and the differentiation from other causes of hyponatraemia is the first essential step in The previous article in this supplement established determining appropriate treatment. Diagnostic criteria that hyponatraemia due to syndrome of inappropriate for SIADH are included in Table 1 (10). In this article, antidiuretic hormone (SIADH) is associated with we review the existing treatments available for the significant morbidity (1), mortality (2–5) and increased management of SIADH and discuss the potential impact length of hospital stay (6). -
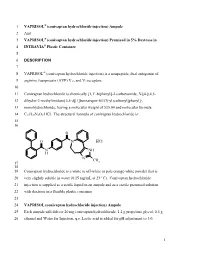
1 VAPRISOL (Conivaptan Hydrochloride Injection)
1 VAPRISOL® (conivaptan hydrochloride injection) Ampule 2 And 3 VAPRISOL® (conivaptan hydrochloride injection) Premixed in 5% Dextrose in 4 INTRAVIA® Plastic Container 5 6 DESCRIPTION 7 8 VAPRISOL® (conivaptan hydrochloride injection) is a nonpeptide, dual antagonist of 9 arginine vasopressin (AVP) V1A and V2 receptors. 10 11 Conivaptan hydrochloride is chemically [1,1’-biphenyl]-2-carboxamide, N-[4-[(4,5- 12 dihydro-2-methylimidazo[4,5-d][1]benzazepin-6(1H)-yl)carbonyl]phenyl]-, 13 monohydrochloride, having a molecular weight of 535.04 and molecular formula 14 C32H26N4O2·HCl. The structural formula of conivaptan hydrochloride is: 15 16 O O N . HCl N NH H N CH 17 3 18 19 Conivaptan hydrochloride is a white to off-white or pale orange-white powder that is 20 very slightly soluble in water (0.15 mg/mL at 23° C). Conivaptan hydrochloride 21 injection is supplied as a sterile liquid in an ampule and as a sterile premixed solution 22 with dextrose in a flexible plastic container. 23 24 VAPRISOL (conivaptan hydrochloride injection) Ampule 25 Each ampule will deliver 20 mg conivaptan hydrochloride, 1.2 g propylene glycol, 0.4 g 26 ethanol and Water for Injection, q.s. Lactic acid is added for pH adjustment to 3.0. 1 27 28 VAPRISOL (conivaptan hydrochloride injection) Premixed in 5% Dextrose 29 Each container contains a clear, colorless, sterile, non-pyrogenic solution of conivaptan 30 hydrochloride in dextrose. Each 100 mL, single-use premixed INTRAVIA Container 31 contains 20 mg of conivaptan hydrochloride and 5 g of Dextrose Hydrous, USP. Lactic 32 Acid, USP is added for pH adjustment to pH 3.4 to 3.8. -

Queensland Health List of Approved Medicines
Queensland Health List of Approved Medicines Drug Form Strength Restriction abacavir * For use in accord with PBS Section 100 indications * oral liquid See above 20 mg/mL See above tablet See above 300 mg See above abacavir + lamivudine * For use in accord with PBS Section 100 indications * tablet See above 600 mg + 300 mg See above abacavir + lamivudine + * For use in accord with PBS Section 100 indications * zidovudine tablet See above 300 mg + 150 mg + 300 mg See above abatacept injection 250 mg * For use in accord with PBS Section 100 indications * abciximab (a) Interventional Cardiologists for complex angioplasty (b) Interventional and Neuro-interventional Radiologists for rescue treatment of thromboembolic events that occur during neuroendovascular procedures. * Where a medicine is not TGA approved, patients should be made fully aware of the status of the medicine and appropriate consent obtained * injection See above 10 mg/5 mL See above abiraterone For use by medical oncologists as per the PBS indications for outpatient and discharge use only tablet See above 250 mg See above 500 mg See above acamprosate Drug and alcohol treatment physicians for use with a comprehensive treatment program for alcohol dependence with the goal of maintaining abstinence. enteric tablet See above 333 mg See above acarbose For non-insulin dependent diabetics with inadequate control despite diet; exercise and maximal tolerated doses of other anti-diabetic agents tablet See above 50 mg See above 100 mg See above acetazolamide injection 500 mg tablet 250 mg acetic acid ear drops 3% 15mL solution 2% 100mL green 3% 1 litre 6% 1 Litre 6% 200mL Generated on: 30-Aug-2021 Page 1 of 142 Drug Form Strength Restriction acetylcysteine injection For management of paracetamol overdose 2 g/10 mL See above 6 g/30 mL See above aciclovir cream Infectious disease physicians, haematologists and oncologists 5% See above eye ointment For use on the advice of Ophthalmologists only.