Joimsi LOWRY
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

'Judas Priest-On Tour' Violators Attacked
MARCH 18, 1978 VOL. 1, NO. 1 Benefiting FREE San Antonio For Your Austin• Houston Entertainment ~ 'Judas Priest-On Tour' JC 35296 The high priest of heavy rock 'n' roll with their inimitable style grace our shores once again. To change or not to change. That is what a rock and roll band must deal with. A group may develop a success ful formula for its music, which leads to personal and/ or commercial contentment. Musicians, upon reach ing this point, find their music evolving in a new direction or continuing their successful format. Judas Priest has choosen the security of proven success. Their first two ViolatorsAttacked domestic albums were well See story on page 10 received in this area. With the release of a new album "Stained Class" and • Elvis Costello an upcoming concert March INSIDETHIS • Radio Survey 24 their claim to fame is • Trivia Quiz sound. ISSUE! .-HELLO IT'SUS- / Welcome to It's Onlu Rock and , Muhammad Ali, chicken fried steak, Roll. What are you being welcomed cars with dead batteries, Rocky Hor to anyway? ror Picture Show and working over It's Only Rock and Roll is a time to afford concert tickets and newspaper/magazine of sorts put out vinyl habits. by a few people who know and love Sound comp'iicated, si1ly, insane, music and believe it's time for a unclear? It is all that and more. semi-intelligent, semi-informed rag Best of all it's fun and we' 11 attanpt about music on the local scene. to write about it: show pictures of Because no one is adequately it and make a meager living from it filling the music news and informa as long as it stays complicated, tion void in San Antonio, we decided silly, insane, unclear and fun. -

Record World
record tWODedicated To Serving The Needs Of The Music & Record Industry x09664I1Y3 ONv iS Of 311VA ZPAS 3NO2 ONI 01;01 3H1 June 21, 1970 113N8V9 NOti 75c - In the opinion of the eUILIRS,la's wee% Tne itmuwmg diCtie SINGLE PICKS OF THE WEEK EPIC ND ARMS CAN EVER HOLD YOU CORBYVINTON Kenny Rogers and the First Mary Hopkin sings Doris Nilsson has what could be Bobby Vinton gets justa Edition advise the world to Day'sphilosophicaloldie, his biggest, a n dpossibly littlenostalgicwith"No "TellItAll Brother" (Sun- "Que Sera, Sera (Whatever hisbest single,a unique Arms Can Ever Hold You" beam, BMI), and it's bound WillBe, WillBe)" (Artist, dittyofhis own devising, (Gil, BMI),whichsounds tobe theirlatestsmash ASCAP), her way (A p p le "Down to the Valley" (Sun- just right for the summer- (Reprise 0923). 1823). beam, BM!) (RCA 74-0362). time (Epic 5-10629). SLEEPER PICKS OF THE WEEK SORRY SUZANNE (Jan-1111 Auto uary, Embassy BMI) 4526 THE GLASS BOTTLE Booker T. & the M. G.'s do Canned Heat do "Going Up The Glass Bottle,a group JerryBlavat,Philly'sGea something just alittledif- the Country" (Metric, BMI) ofthreeguysandthree for with the Heater,isal- ferent,somethingjust a astheydo itin"Wood- gals, introduce themselves ready clicking with "Tasty littleliltingwith"Some- stock," and it will have in- with "Sorry Suzanne" (Jan- (To Me),"whichgeators thing" (Harrisongs, BMI) creasedmeaningto the uary, BMI), a rouser (Avco andgeatoretteswilllove (Stax 0073). teens (Liberty 56180). Embassy 4526). (Bond 105). ALBUM PICKS OF THE WEEK DianaRosshasherfirst "Johnny Cash the Legend" "The Me Nobody Knows" "The Naked Carmen" isa solo album here, which is tributed in this two -rec- isthe smash off-Broadway "now"-ized versionofBi- startsoutwithherfirst ord set that includes' Fol- musical adaptation of the zet's timeless opera. -

Omega Auctions Ltd Catalogue 28 Apr 2020
Omega Auctions Ltd Catalogue 28 Apr 2020 1 REGA PLANAR 3 TURNTABLE. A Rega Planar 3 8 ASSORTED INDIE/PUNK MEMORABILIA. turntable with Pro-Ject Phono box. £200.00 - Approximately 140 items to include: a Morrissey £300.00 Suedehead cassette tape (TCPOP 1618), a ticket 2 TECHNICS. Five items to include a Technics for Joe Strummer & Mescaleros at M.E.N. in Graphic Equalizer SH-8038, a Technics Stereo 2000, The Beta Band The Three E.P.'s set of 3 Cassette Deck RS-BX707, a Technics CD Player symbol window stickers, Lou Reed Fan Club SL-PG500A CD Player, a Columbia phonograph promotional sticker, Rock 'N' Roll Comics: R.E.M., player and a Sharp CP-304 speaker. £50.00 - Freak Brothers comic, a Mercenary Skank 1982 £80.00 A4 poster, a set of Kevin Cummins Archive 1: Liverpool postcards, some promo photographs to 3 ROKSAN XERXES TURNTABLE. A Roksan include: The Wedding Present, Teenage Fanclub, Xerxes turntable with Artemis tonearm. Includes The Grids, Flaming Lips, Lemonheads, all composite parts as issued, in original Therapy?The Wildhearts, The Playn Jayn, Ween, packaging and box. £500.00 - £800.00 72 repro Stone Roses/Inspiral Carpets 4 TECHNICS SU-8099K. A Technics Stereo photographs, a Global Underground promo pack Integrated Amplifier with cables. From the (luggage tag, sweets, soap, keyring bottle opener collection of former 10CC manager and music etc.), a Michael Jackson standee, a Universal industry veteran Ric Dixon - this is possibly a Studios Bates Motel promo shower cap, a prototype or one off model, with no information on Radiohead 'Meeting People Is Easy 10 Min Clip this specific serial number available. -

Toe Fat Two Mp3, Flac, Wma
Toe Fat Two mp3, flac, wma DOWNLOAD LINKS (Clickable) Genre: Rock / Folk, World, & Country Album: Two Country: Germany Released: 2005 Style: Hard Rock, Prog Rock MP3 version RAR size: 1869 mb FLAC version RAR size: 1626 mb WMA version RAR size: 1164 mb Rating: 4.1 Votes: 931 Other Formats: DTS AU VOC ADX VQF DXD DMF Tracklist 1 Stick Heat 2 Indian Summer 3 Idol 4 There'll Be Changes 5 A New Way 6 Since You've Been Gone 7 Three Time Loser 8 Midnight Sun 9 Brand New Band 10 Can't Live Without You Credits Bass Guitar, Vocals – John Konas* Drums, Vocals – Brian Glascock Electric Guitar, Acoustic Guitar – Alan Kendall Engineer – John Barratt, Peter Mew Flute, Harmonica – Mox* Lead Vocals – Cliff Bennett Photography By, Design [Cover Design] – Hipgnosis Producer – Jonathan Peel Written-By – Alan Kendall, Cliff Bennett (tracks: A1, A4 to B4) Barcode and Other Identifiers Barcode: 4009910441725 Other versions Category Artist Title (Format) Label Category Country Year SLRZ 1015, 1E Regal Zonophone, SLRZ 1015, 1E Toe Fat Two (LP, Album) UK 1970 062-04575 EMI 062-04575 Two (LP, Album, Ltd, 03501 Toe Fat Soundvision 03501 Europe 2013 RE, Red) Two (CD, Album, RE, Repertoire REP 4417-WY Toe Fat REP 4417-WY Russia 2016 Unofficial) Records Repertoire REP 4417-WY Toe Fat Two (CD, Album, RE) REP 4417-WY Germany 1994 Records Two (LP, Album, Ltd, 03501 Toe Fat Soundvision 03501 Europe 2013 RE, Blu) Related Music albums to Two by Toe Fat Bruce Pratt & The Iron Horse Band - Raton Sunset The Bee Gees - Life In A Tin Can Cliff Bennett - Memphis Streets Kate Bush - Never For Ever Kate Maki - Head In The Sand Bronco - Ace Of Sunlight The Alan Hull Songbook - Some Other Time Cosy Sheridan - Grand Design Alan D. -

Rules for Students/Wlogo
The Ten Rules of Concert Etiquette www.musicfriends.org (For Students) 1 Refrain from talking The first and greatest rule. It also includes whispering during the music. 2 No singing, tapping fingers or feet The musicians do not need your help, and your neighbors need silence. Learn to tap your toes quietly inside your shoes– it’s a good exercise to reduce toe fat. 3 Please have nothing in your mouth, besides your teeth and tongue Gum and candy are not allowed. 4 Do not wear watches with alarms nor jangle jewelry You may enjoy the sound, but the added percussion is disturbing to everyone around you. 5 Do not open and close your purse nor rip open your velcro wallet The best plan is to leave purses, etc., back at school or on the locked bus. 6 Do not sigh with boredom If you are in agony, keep it to yourself. Your neighbor just may be in ecstasy, which should also be kept under control. 7 Do not applaud between movements You may think the music is over, but it is not. You don’t want to be the only one clapping. 8 Do not embarrass your teacher nor your school Remember that you are representing your school, and you want to be on your best behavior. There are many eyes looking at you. 9 Do not read nor play with a toy in your pocket To listen means just that. Use the time to turn on a “video screen” in your mind and create a story to the music. -

Music & Entertainment
Hugo Marsh Neil Thomas Forrester Director Shuttleworth Director Director Music & Entertainment Tuesday 17th & Wednesday 18th November 2020 at 10:00 Viewing on a rota basis by appointment only For enquires relating to the Special Auction Services auction, please contact: Plenty Close Off Hambridge Road NEWBURY RG14 5RL Telephone: 01635 580595 Email: [email protected] www.specialauctionservices.com David Martin Dave Howe Music & Music & Entertainment Entertainment Due to the nature of the items in this auction, buyers must satisfy themselves concerning their authenticity prior to bidding and returns will not be accepted, subject to our Terms and Conditions. Additional images are available on request. Buyers Premium with SAS & SAS LIVE: 20% plus Value Added Tax making a total of 24% of the Hammer Price the-saleroom.com Premium: 25% plus Value Added Tax making a total of 30% of the Hammer Price 4. Touch LP, Touch - Original UK 10. Progressive Rock LPs, twelve DAY ONE Mono Release 1969 on Deram (DML 1033) albums of mainly Classic and Prog - With Poster - Laminated Gatefold Garrod Rock comprising The Who (Tommy and & Lofthouse Sleeve - Original Mono Inner Quadrophenia), Led Zeppelin II, Deep Vinyl Records - Brown / White Labels - Sleeve, Poster, Purple (Burn and Concerto For Group and Inner and Vinyl all in Excellent condition Orchestra), Black Sabbath - Sabbath Bloody A large number of the original albums £60-100 Sabbath, Pink Floyd - Ummagumma, David and singles in the Vinyl section make Bowie - 1980 All Clear (Promo), Osibisa -

YTAA WUDR 06-01-2021 Memorial Show Wsg Tom Gilliam Set 1 1 Dr
YTAA WUDR 06-01-2021 Memorial Show wsg Tom Gilliam Set 1 1 Dr. J New WUDR legal ID 0:27 Art Jipson ---- 2 Keith Harrison YTAA Show ID 0:19 YTAA Show IDs Keith Harrison ---- 3 The Ballroom Blitz 4:04 The Best of Sweet The Sweet Steve Priest 4 Tutti Frutti 2:27 Little Richard's Greatest Hits Little Richard Little Richard 5 That Thing You Do 2:53 That Thing You Do Soundtrack Wonders Adam Schlesinger 6 Because the Night 3:25 Easter Patti Smith Group Ivan Kral – PSG Discuss Priest, Little Richard and Kral Set 2 7 I Remember Everything 2:43 I Remember Everything - Single John Prine John Prine 8 Your Love 3:43 Big Innings: Best Of The Outfield The Outfield Tony Lewis 9 Mexican Wine 3:22 Welcome Interstate Managers Fountains Of Wayne Adam Schlesinger 10 I Can See Clearly Now 2:43 Radio Hits of the '70s Johnny Nash Johnny Nash Discuss Prine, Lewis and Nash Set 3 11 Man-Sized Wreath 2:33 Accelerate R.E.M. Bill Rieflin (REM, King Crim) 12 Small Town Saturday Night 2:56 Hal Ketchum: The Hits Hal Ketchum Hal Ketchum 13 Stacy's Mom 3:18 Welcome Interstate Managers Fountains Of Wayne Adam Schlesinger 14 Lovely Day 4:17 Menagerie Bill Withers Bill Withers Discuss Rieflin, Kethcum, Schlesinger Set 4 15 Fade Into You 4:56 So Tonight That I Might See Mazzy Star David Roback 16 Standing In the Doorway 7:43 Time Out of Mind Bob Dylan Bucky Baxter (pedal steel, Dylan) 17 The Big Money (Neil Peart) 5:37 Power Windows Rush Neil Pert 18 Hackensack 3:00 Welcome Interstate Managers Fountains Of Wayne Adam Schlesinger Discuss Roback, Baxter, Pert, Schlesinger Set 5 19. -

Hallowed PDF-Article Design by Daniel Källmalm Hallowed PDF
Hallowed PDF-article Hallowed PDF-article Design by Daniel Källmalm Design by Daniel Källmalm Hallowed PDF-article Hallowed PDF-article Design by Daniel Källmalm Design by Daniel Källmalm subsequently became Vertigo. During the following months after beginning to work with Bron, the band changed the name to Uriah Heep (the ‘orrible little character from David Copperfield by Charles Dickens who was very much in fa- shion then because of the hundredth year since his death) on suggestion from Bron; they also started using the keyboards in their music. - We’d actually recorded half the first album when we decided that keyboards would be good for our sound, says Box who got this inspi- ration somewhat from being a fan of Vanilla Fudge with their hammond organs with guitars soaring on top. They decided to shape it like that along with David’s vibrato vocals. First they had session keyboardist Colin Woods who was brought in by Bron and it was when they started looking for a permanent keyboardist they found Ken Hensley who had been playing with Newton in The Gods and was now playing the gui- tar for Toe Fat. Hensley explains that he saw the potential to do something different with Uriah Heep which The history of Uriah Heep can Having higher ambitions that their though at first that made it difficult is why he joined the band. And it be dated all the way back to fellow band members Mick Box and getting gigs, eventually we built a was not only Hensley’s talents as a 1967, the year after the English David Byron quit their day jobs to little cult following because of that. -

Ken Hensley Discography
THE COMPLETE KEN HENSLEY DISCOGRAPHY DISCOGRAPHY (UK) Page 2 ALBUMS Page 3 SINGLES Page 7 LINE-UPS & SESSIONS Page 8 SONGS ALPHABETICALLY Page 10 SONGS CHRONOLOGICALLY Page 13 DIFFERENT VERSIONS OF SONGS Page 16 Compiled by Tapio Minkkinen With help from Stay On Top, The Official Uriah Heep Appreciation Society, Roy Landgren and other Heep fans too many to mention here & © 1996 - 2020 Tapio Minkkinen. For personal use only. Do not copy or distribute without permission. K E N H E N S L E Y D I S C O G R A P H Y 1 9 6 7 - 2 0 2 0 & © 1996 - 2020 Tapio Minkkinen. www.heepfiles.net . For personal use only. Do not copy or distribute without permission. DISCOGRAPHY (U.K.) This is a selective discography. UK releases unless otherwise stated. Other releases have been included if they differ from UK ones. THE GODS Come On Down To My Boat Baby / Garage Man S 1967 Polydor 56168 GENESIS LP 1968 Columbia SX 6286 Baby’s Rich / Somewhere In The Street S 1968 Columbia DB 8486 Hey Bulldog / Real Love Guaranteed S 1969 Columbia DB 8544 Maria / Long Time, Sad Time, Bad Time S 1969 Columbia DB 8572 TO SAMUEL A SON LP 1970 Columbia SX 6372 FEATURING KEN HENSLEY collection LP 1976 Columbia SHSM 2011 THE VERY BEST collection LP 1989 C5 C5 537 HEAD MACHINE ORGASM LP 1970 Major Minor SMLP 79 CLIFF BENNETT BAND Memphis Streets / But I’m Wrong S 1970 Parlophone 5792 TOE FAT TOE FAT LP 1970 Parlophone PCS 7090 Bad Side Of The Moon / Working Nights S 1970 Parlophone 5829 Just Like Me / Bad Side Of The Moon S US 1970 Rare Earth 5019 WEED WEED…! LP GER 1971 Philips -
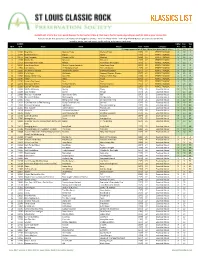
KLASSICS LIST Criteria
KLASSICS LIST criteria: 8 or more points (two per fan list, two for U-Man A-Z list, two to five for Top 95, depending on quartile); 1984 or prior release date Sources: ten fan lists (online and otherwise; see last page for details) + 2011-12 U-Man A-Z list + 2014 Top 95 KSHE Klassics (as voted on by listeners) sorted by points, Fan Lists count, Top 95 ranking, artist name, track name SLCRPS UMan Fan Top ID # ID # Track Artist Album Year Points Category A-Z Lists 95 35 songs appeared on all lists, these have green count info >> X 10 n 1 12404 Blue Mist Mama's Pride Mama's Pride 1975 27 PERFECT KLASSIC X 10 1 2 12299 Dead And Gone Gypsy Gypsy 1970 27 PERFECT KLASSIC X 10 2 3 11672 Two Hangmen Mason Proffit Wanted 1969 27 PERFECT KLASSIC X 10 5 4 11578 Movin' On Missouri Missouri 1977 27 PERFECT KLASSIC X 10 6 5 11717 Remember the Future Nektar Remember the Future 1973 27 PERFECT KLASSIC X 10 7 6 10024 Lake Shore Drive Aliotta Haynes Jeremiah Lake Shore Drive 1971 27 PERFECT KLASSIC X 10 9 7 11654 Last Illusion J.F. Murphy & Salt The Last Illusion 1973 27 PERFECT KLASSIC X 10 12 8 13195 The Martian Boogie Brownsville Station Brownsville Station 1977 27 PERFECT KLASSIC X 10 13 9 13202 Fly At Night Chilliwack Dreams, Dreams, Dreams 1977 27 PERFECT KLASSIC X 10 14 10 11696 Mama Let Him Play Doucette Mama Let Him Play 1978 27 PERFECT KLASSIC X 10 15 11 11547 Tower Angel Angel 1975 27 PERFECT KLASSIC X 10 19 12 11730 From A Dry Camel Dust Dust 1971 27 PERFECT KLASSIC X 10 20 13 12131 Rosewood Bitters Michael Stanley Michael Stanley 1972 27 PERFECT -

The Official Appreciation Society Issue 7
THE OFFICIAL APPRECIATION SOCIETY ISSUE7 lHE CF;iCI^L THE OFFiCIAL URIAHHEEP APPRECIAIIONSOCiE?Y P.O.SOX258 iELFCRD SHROPSHiRE TF2 5JG APPI?ECIATIONSOC|F|Y ENGi,AiID URI,AHHE:PS OFFICIALWORLD WDE FANCLUB - ADMINISTEFEDgY DAVIDO\A/EN & ALANHARTLEY Hi againand welcometo issue 7, lknow it's March bui as ihis is the firsl issue o{ 1993 we want lo wish all our msmbers the very best lor ihe year. Not only a new year but also a new look magazine, il you're worried that the smailsr size means less inlormation then le'i us assure you thal it doesn'l. The size oi the print is halt that ot the old style magazine so lhere is exactly the same amount o{ Heep inlormation on each page" So, why have we changed i't,well, the magazine has sleadi!y increased in size wirh exlra pages being added on a regular basis, the reduclion in size allows us to carry on doing this within our present budget. That can only be good lor sverycne so we hope you will gel even more enjoyment lrom this issue. You're ali probabiy waiting to read il there's baen any new developments on ih€ "Lrve new recording deal and lhe release ol the In Moscow" video. There's an exctusiveinierview with Mick in this issue and he gives us the latest in{ormation on these and other points so we'll let you read all aboui il lhere. The German lour with Nazareth has been s great success bLrt the dates did change somewhat to lhose reported in issue 6 Onc.e again the last conc€rl in Osnabrock on 4lh March was lull ol surprises but Heep really out diC Nazareth. -

EURASIA MAILORDER British List 2021/8/2 Updated How to Order
EURASIA MAILORDER British List 2021/9/1 updated How to Order 10CC [HOW DARE YOU!] (UK / MERCURY / 75 / FOC/WI) EX/EX USD 16 / EURO14 10CC [SAME] (UK / UK RECORD / 74 / WI) EX/EX USD 25 / EURO21 10CC [SHEET MUSIC] (UK / UK RECORD / 74 / WI) EX/EX USD 23 / EURO19 11.59 [THIS OUR SACRIFICE OF PRAISE] (UK / DOVETAIL / 74 / WI) EX+/EX USD 180 / EURO152 9.30 FLY [SAME] (UK / EMBER / 72 / FOC) EX+/EX USD 342 / EURO288 ABSOLUTE ELSEWHERE [IN SEARCH OF ANCIENT GODS] (UK / WB / 76 / GIMC/WB) EX/EX USD 61 / EURO52 ABSOLUTE ELSEWHERE [IN SEARCH OF ANCIENT GODS] (UK / WB / 76 / GIMC/WB/SOC) EX/EX USD 34 / EURO29 ABSOLUTE ELSEWHERE [IN SEARCH OF ANCIENT GODS] (UK / WB / 76 / GIMC/WB) EX+/EX USD 52 / EURO44 ACHOR AND FRIENDS [HOSANNA TO THE SON OF DAVID] ( / / / ) EX+/EX+ USD 52 / EURO44 ADRIAN WAGNEFR [DISTANCES BETWEEN US] (UK / ATLANTIC / 74 / ) M-/EX USD 43 / EURO36 ADRIAN WAGNEFR [INSTINCTS] (UK / CHARISMA / 77 / ) EX/EX USD 34 / EURO29 AFFINITY [SAME] (ITA / AKARMA / / FOC) M-/M- USD 34 / EURO29 AFTER THE FIRE [SIGNS OF CHANGE] (UK / RAPID / 78 / WI) EX/EX USD 144 / EURO121 AFTER THE FIRE [SIGNS OF CHANGE] (UK / RAPID / 78 / ) EX/EX USD 108 / EURO91 AL ET AL [STRANGE AFFER] (UK / ARNY'S SHACK / / ) VG/VG+ USD 252 / EURO212 ALAN BOWN [STRETCHING OUT] (UK / ISLAND / 71 / FOC) EX/EX USD 34 / EURO29 ALAN BOWN [STRETCHING OUT] (UK / ISLAND / 71 / FOC) EX/EX USD 25 / EURO21 ALAN COHEN BAND [BLACK BROWN & BEIGE] (UK / ARGO / 73 / WI) EX/EX USD 90 / EURO76 ALAN SKIDMORE QUINTET [TCB] (UK / PHILIPS / 70 / ) EX+/EX+ USD 295 / EURO248 ALEANNA [SAME] (UK /