Have Bird Distributions Shifted Along an Elevational Gradient on a Tropical Mountain?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bird Checklist Guánica Biosphere Reserve Puerto Rico
United States Department of Agriculture BirD CheCklist Guánica Biosphere reserve Puerto rico Wayne J. Arendt, John Faaborg, Miguel Canals, and Jerry Bauer Forest Service Research & Development Southern Research Station Research Note SRS-23 The Authors: Wayne J. Arendt, International Institute of Tropical Forestry, U.S. Department of Agriculture Forest Service, Sabana Field Research Station, HC 2 Box 6205, Luquillo, PR 00773, USA; John Faaborg, Division of Biological Sciences, University of Missouri, Columbia, MO 65211-7400, USA; Miguel Canals, DRNA—Bosque de Guánica, P.O. Box 1185, Guánica, PR 00653-1185, USA; and Jerry Bauer, International Institute of Tropical Forestry, U.S. Department of Agriculture Forest Service, Río Piedras, PR 00926, USA. Cover Photos Large cover photograph by Jerry Bauer; small cover photographs by Mike Morel. Product Disclaimer The use of trade or firm names in this publication is for reader information and does not imply endorsement by the U.S. Department of Agriculture of any product or service. April 2015 Southern Research Station 200 W.T. Weaver Blvd. Asheville, NC 28804 www.srs.fs.usda.gov BirD CheCklist Guánica Biosphere reserve Puerto rico Wayne J. Arendt, John Faaborg, Miguel Canals, and Jerry Bauer ABSTRACt This research note compiles 43 years of research and monitoring data to produce the first comprehensive checklist of the dry forest avian community found within the Guánica Biosphere Reserve. We provide an overview of the reserve along with sighting locales, a list of 185 birds with their resident status and abundance, and a list of the available bird habitats. Photographs of habitats and some of the bird species are included. -

Rainfall Correlates of Bird Population Fluctuations in a Puerto Rican Dry Forest: a Nine Year Study
WilsonBull., 96(4), 1984,pp. 575-593 RAINFALL CORRELATES OF BIRD POPULATION FLUCTUATIONS IN A PUERTO RICAN DRY FOREST: A NINE YEAR STUDY JOHN FAABORG, WAYNE J. ARENDT, AND MARK S. KAISER Long-term studies on the population dynamics of Neotropical bird communities have been primarily limited to Panama (see Karr et al. [ 19821 for a mainland site and Willis [1974] for Barro Colorado Island). An earlier paper (Faaborg 1982a) contained the first long-term population measurements from a West Indian island, specifically a seasonally-dry forest site in southwest Puerto Rico. This Syear study apparently spanned a population peak followed by drought conditions and a severe population decline. The effects of drought on total populations, membership in dif- ferent foraging guilds, and winter resident densities were discussed. We have continued these studies and here report on 9 years of banding and population monitoring activities in a single location. This allows us to expand our previous observations on relationships between rainfall patterns and population traits of guilds and species and expose the data to statistical analyses. We also document the attempted invasion of a new species (Elaenia martinica) into the Guanica Forest bird community. The possible meaning of these observations in terms of island equilibrium theory (MacArthur and Wilson 1967), long-term climatic patterns (Pregill and Olson 198 l), and community structure studies (Faaborg 1982b) is discussed. STUDY AREA AND METHODS This study was done in seasonallydry scrubin the Guanica Forest of southwesternPuerto Rico (see Terborgh and Faaborg [1973] for detailed habitat description with photographs). This habitat occurs on a coraline limestone and contains sclerophyllousforest typical of such sites throughout the West Indies (Beard 1949). -

Juan Cristóbal Gundlach's Collections of Puerto Rican Birds with Special
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Zoosystematics and Evolution Jahr/Year: 2015 Band/Volume: 91 Autor(en)/Author(s): Frahnert Sylke, Roman Rafela Aguilera, Eckhoff Pascal, Wiley James W. Artikel/Article: Juan Cristóbal Gundlach’s collections of Puerto Rican birds with special regard to types 177-189 Creative Commons Attribution 4.0 licence (CC-BY); original download https://pensoft.net/journals Zoosyst. Evol. 91 (2) 2015, 177–189 | DOI 10.3897/zse.91.5550 museum für naturkunde Juan Cristóbal Gundlach’s collections of Puerto Rican birds with special regard to types Sylke Frahnert1, Rafaela Aguilera Román2, Pascal Eckhoff1, James W. Wiley3 1 Museum für Naturkunde, Leibniz-Institut für Evolutions- und Biodiversitätsforschung, Invalidenstraße 43, D-10115 Berlin, Germany 2 Instituto de Ecología y Sistemática, La Habana, Cuba 3 PO Box 64, Marion Station, Maryland 21838-0064, USA http://zoobank.org/B4932E4E-5C52-427B-977F-83C42994BEB3 Corresponding author: Sylke Frahnert ([email protected]) Abstract Received 1 July 2015 The German naturalist Juan Cristóbal Gundlach (1810–1896) conducted, while a resident Accepted 3 August 2015 of Cuba, two expeditions to Puerto Rico in 1873 and 1875–6, where he explored the Published 3 September 2015 southwestern, western, and northeastern regions of this island. Gundlach made repre sentative collections of the island’s fauna, which formed the nucleus of the first natural Academic editor: history museums in Puerto Rico. When the natural history museums closed, only a few Peter Bartsch specimens were passed to other institutions, including foreign museums. None of Gund lach’s and few of his contemporaries’ specimens have survived in Puerto Rico. -

Eleutherodactylus Coqui Global Invasive Species Database (GISD)
FULL ACCOUNT FOR: Eleutherodactylus coqui Eleutherodactylus coqui System: Freshwater_terrestrial Kingdom Phylum Class Order Family Animalia Chordata Amphibia Anura Leptodactylidae Common name coqui (German), Puerto Rican treefrog (English, Puerto Rico), Caribbean tree frog (English), common coqui (English) Synonym Similar species Eleutherodactylus planirostris, Eleutherodactylus martinicensis Summary Eleutherodactylus coqui is a relatively small tree frog native to Puerto Rico. The frogs are quite adaptable to different ecological zones and elevations. Their loud call is the main reason they are considered a pest. E. coqui's mating call is its namesake, a high- pitched, two-note \"co-qui\" (ko-kee') which attains nearly 100 decibels at 0.5 metres. E. coqui have a voracious appetite and there is concern in Hawai‘i, where it has been introduced, that E. coqui may put Hawai‘i’s endemic insect and spider species at risk and compete with endemic birds and other native fauna which rely on insects for food. view this species on IUCN Red List Species Description Eleutherodactylus coqui is described as a relatively small treefrog. In Puerto Rico, mature calling males and \"parental males\" (males guarding a clutch) average about 34mm in length from snout to vent (snout-vent length, or SVL), while mature egg-laying females average about 41mm SVL. Like the true treefrogs (family Hylidae), E. coqui have well developed pads at the end of each toe that are used for sticking to surfaces. E. coqui are extremely variable in colouration. The dorsum (upper surface) is generally grey or grey-brown and uniform in colour, or may have either a dark \"M\" shape between the shoulders, two broad, light dorso-lateral bars (from the snout, through to the eye, to the axila of the rear legs) bordered with black spots and/or a light bar on top of the head between the eyes and a light underside stippled with brown (Campbell, 2000). -

Distribution, Ecology, and Life History of the Pearly-Eyed Thrasher (Margarops Fuscatus)
Adaptations of An Avian Supertramp: Distribution, Ecology, and Life History of the Pearly-Eyed Thrasher (Margarops fuscatus) Chapter 6: Survival and Dispersal The pearly-eyed thrasher has a wide geographical distribution, obtains regional and local abundance, and undergoes morphological plasticity on islands, especially at different elevations. It readily adapts to diverse habitats in noncompetitive situations. Its status as an avian supertramp becomes even more evident when one considers its proficiency in dispersing to and colonizing small, often sparsely The pearly-eye is a inhabited islands and disturbed habitats. long-lived species, Although rare in nature, an additional attribute of a supertramp would be a even for a tropical protracted lifetime once colonists become established. The pearly-eye possesses passerine. such an attribute. It is a long-lived species, even for a tropical passerine. This chapter treats adult thrasher survival, longevity, short- and long-range natal dispersal of the young, including the intrinsic and extrinsic characteristics of natal dispersers, and a comparison of the field techniques used in monitoring the spatiotemporal aspects of dispersal, e.g., observations, biotelemetry, and banding. Rounding out the chapter are some of the inherent and ecological factors influencing immature thrashers’ survival and dispersal, e.g., preferred habitat, diet, season, ectoparasites, and the effects of two major hurricanes, which resulted in food shortages following both disturbances. Annual Survival Rates (Rain-Forest Population) In the early 1990s, the tenet that tropical birds survive much longer than their north temperate counterparts, many of which are migratory, came into question (Karr et al. 1990). Whether or not the dogma can survive, however, awaits further empirical evidence from additional studies. -

Puerto Rico Comprehensive Wildlife Conservation Strategy 2005
Comprehensive Wildlife Conservation Strategy Puerto Rico PUERTO RICO COMPREHENSIVE WILDLIFE CONSERVATION STRATEGY 2005 Miguel A. García José A. Cruz-Burgos Eduardo Ventosa-Febles Ricardo López-Ortiz ii Comprehensive Wildlife Conservation Strategy Puerto Rico ACKNOWLEDGMENTS Financial support for the completion of this initiative was provided to the Puerto Rico Department of Natural and Environmental Resources (DNER) by U.S. Fish and Wildlife Service (USFWS) Federal Assistance Office. Special thanks to Mr. Michael L. Piccirilli, Ms. Nicole Jiménez-Cooper, Ms. Emily Jo Williams, and Ms. Christine Willis from the USFWS, Region 4, for their support through the preparation of this document. Thanks to the colleagues that participated in the Comprehensive Wildlife Conservation Strategy (CWCS) Steering Committee: Mr. Ramón F. Martínez, Mr. José Berríos, Mrs. Aida Rosario, Mr. José Chabert, and Dr. Craig Lilyestrom for their collaboration in different aspects of this strategy. Other colleagues from DNER also contributed significantly to complete this document within the limited time schedule: Ms. María Camacho, Mr. Ramón L. Rivera, Ms. Griselle Rodríguez Ferrer, Mr. Alberto Puente, Mr. José Sustache, Ms. María M. Santiago, Mrs. María de Lourdes Olmeda, Mr. Gustavo Olivieri, Mrs. Vanessa Gautier, Ms. Hana Y. López-Torres, Mrs. Carmen Cardona, and Mr. Iván Llerandi-Román. Also, special thanks to Mr. Juan Luis Martínez from the University of Puerto Rico, for designing the cover of this document. A number of collaborators participated in earlier revisions of this CWCS: Mr. Fernando Nuñez-García, Mr. José Berríos, Dr. Craig Lilyestrom, Mr. Miguel Figuerola and Mr. Leopoldo Miranda. A special recognition goes to the authors and collaborators of the supporting documents, particularly, Regulation No. -

Guide to Theecological Systemsof Puerto Rico
United States Department of Agriculture Guide to the Forest Service Ecological Systems International Institute of Tropical Forestry of Puerto Rico General Technical Report IITF-GTR-35 June 2009 Gary L. Miller and Ariel E. Lugo The Forest Service of the U.S. Department of Agriculture is dedicated to the principle of multiple use management of the Nation’s forest resources for sustained yields of wood, water, forage, wildlife, and recreation. Through forestry research, cooperation with the States and private forest owners, and management of the National Forests and national grasslands, it strives—as directed by Congress—to provide increasingly greater service to a growing Nation. The U.S. Department of Agriculture (USDA) prohibits discrimination in all its programs and activities on the basis of race, color, national origin, age, disability, and where applicable sex, marital status, familial status, parental status, religion, sexual orientation genetic information, political beliefs, reprisal, or because all or part of an individual’s income is derived from any public assistance program. (Not all prohibited bases apply to all programs.) Persons with disabilities who require alternative means for communication of program information (Braille, large print, audiotape, etc.) should contact USDA’s TARGET Center at (202) 720-2600 (voice and TDD).To file a complaint of discrimination, write USDA, Director, Office of Civil Rights, 1400 Independence Avenue, S.W. Washington, DC 20250-9410 or call (800) 795-3272 (voice) or (202) 720-6382 (TDD). USDA is an equal opportunity provider and employer. Authors Gary L. Miller is a professor, University of North Carolina, Environmental Studies, One University Heights, Asheville, NC 28804-3299. -

Puerto Rico Birding & Nature Tour February 25-29, 2016
P.O. Box 16545 Portal, AZ 85632 Phone 520.558.1146/558.7781 Toll free 800.426.7781 Fax 650.471.7667 Email [email protected] [email protected] Caribbean Islands: Puerto Rico Birding & Nature Tour February 25-29, 2016 Explore Puerto Rico’s prime birding locations where you’ll discover America’s only tropical forests. Seek out 17 endemic bird species in this short, 5-day tour. Indulge yourself in exotic birding where you don’t need a passport; Puerto Rico’s excellent road system allows us explore with ease. Spot an array of Caribbean specialty species, and learn from the expertise of the infectiously enthusiastic guide, Gabriel Lugo, President of the Puerto Rican Ornithological Society. Tour Highlights: - Discover the extremely endangered Puerto Rican Parrot and observe efforts to protect it - Walk cobbled streets of a 400-year old city in Old San Juan - Discover lush mountain forests home to Elfin Woods Warbler and other species - Spot very local and endangered species such as the Yellow-shouldered Blackbird - Explore a variety of habitats: Atlantic and southwest coastlines, the ‘haystack hills’ of the west, dry forests along the Caribbean Sea, and a lush central mountain rainforest. - Relax at a seaside eco-lodge on Puerto Rico’s southern coast Book your tour by e-mailing [email protected] or phone us at toll-free 866.900.1146, worldwide: 520.558.1146 Naturalist Journeys, LLC / Caligo Ventures PO Box 16545 Portal, AZ 85632 PH: 520.558.1146 / 800.426.7781 Fax 650.471.7667www.naturalistjourneys.com / www.caligo.com [email protected] / [email protected] ITINERARY Thursday, Feb 25 ARRIVAL in San Juan Arrive in San Juan, the second oldest Capitol City in the Western Hemisphere. -

The Effects of Hurricanes on Birds, with Special Reference to Caribbean Islands
Bird Conservation International (1993) 3:319-349 The effects of hurricanes on birds, with special reference to Caribbean islands JAMES W. WILEY and JOSEPH M. WUNDERLE, JR. Summary Cyclonic storms, variously called typhoons, cyclones, or hurricanes (henceforth, hurricanes), are common in many parts of the world, where their frequent occurrence can have both direct and indirect effects on bird populations. Direct effects of hurricanes include mortality from exposure to hurricane winds, rains, and storm surges, and geo- graphic displacement of individuals by storm winds. Indirect effects become apparent in the storm's aftermath and include loss of food supplies or foraging substrates; loss of nests and nest or roost sites; increased vulnerability to predation; microclimate changes; and increased conflict with humans. The short-term response of bird populations to hurricane damage, before changes in plant succession, includes shifts in diet, foraging sites or habitats, and reproductive changes. Bird populations may show long-term responses to changes in plant succession as second-growth vegetation increases in storm- damaged old-growth forests. The greatest stress of a hurricane to most upland terrestrial bird populations occurs after its passage rather than during its impact. The most important effect of a hurricane is the destruction of vegetation, which secondarily affects wildlife in the storm's after- math. The most vulnerable terrestrial wildlife populations have a diet of nectar, fruit, or seeds; nest, roost, or forage on large old trees; require a closed forest canopy; have special microclimate requirements and/or live in a habitat in which vegetation has a slow recovery rate. Small populations with these traits are at greatest risk to hurricane-induced extinction, particularly if they exist in small isolated habitat fragments. -

Upcoming Events
Upcoming Events • IAS Fall Festival 2013 10/4/2013 6:00 PM • Another Puerto Rico Trip February 2014, see details inside • Dominican Republic February 2014, see details inside August 2013 | USPS 129-810 Vol. 43, No. 4 | ISSN 0162-8186 Buff-breasted sandpiper photo by Brad Bumgardner The Cardinal is published six times a year by the Indiana Audubon Society, Scott Arvin, Editor, E-mail: [email protected] Drew Lanham include: West Beach, Ogden Dunes, Beverly She was the contemporary of, and good friends October 4-6, 2013 Shores, Mount Baldy, and Michigan City Harbor. with, those shaping America and Indiana’s thoughts on natural history and interpretation. Friday, October 4, 2013 Enos Mills, Col. Charles Lieber and Charles Deam 7:00 PM - BIRD SURVEYS FROM THE INDIANA DUNES STATE PARK were among those she corresponded with DUNES BIRD OBSERVATION TOWER Meet in the main entrance and invited to her home in Rome City, Indiana. PRESENTER - BRAD BUMGARDNER parking lot at 7:00am. She said every bird native to Indiana could be In the past two years, Indiana Dunes State Park found on the grounds of Wildflower Woods! This trip will be one of two birding offerings staff and local volunteers have recorded nearly The Gene Stratton-Porter State Historic Site (dependent upon weather conditions). If a million birds in now-annual spring longshore is currently returning 80 acres of agricultural ideal conditions exist, the group will conduct flight bird surveys. This survey, with federal ground to grasslands and restored wetlands a longshore fall flight watch from atop funding, is transforming our knowledge of to provide additional habitat for birds and the Dunes State Park Beach Pavilion. -
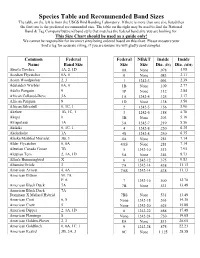
Species Table and Recommended Band Sizes the Table on the Left Is from the USGS Bird Banding Laboratory
Species Table and Recommended Band Sizes The table on the left is from the USGS Bird Banding Laboratory. If there is more than one size listed then the first one is the preferred recommended size. The table on the right may be used to find the National Band & Tag Company butt-end band style that matches the federal band size you are looking for. This Size Chart should be used as a guide only! We cannot be responsible for incorrect sizes being ordered based on this chart. Please measure your bird’s leg for accurate sizing, if you are unsure we will gladly send samples. Common Federal Federal NB&T Inside Inside Name Band Size Size Size Dia. (IN) Dia. (MM) Abert's Towhee 1A, 2, 1D 0A None .078 1.98 Acadian Flycatcher 0A, 0 0 None .083 2.11 Acorn Woodpecker 2, 3 1 1242-3 .094 2.39 Adelaide's Warbler 0A, 0 1B None .109 2.77 Adelie Penguin 9 1P None .112 2.84 African Collared-Dove 3A 1A 1242-4 .125 3.17 African Penguin 9 1D None .138 3.50 African Silverbill 0, 1C, 1 2 1242-5 .156 3.96 Akekee 1B, 1C, 1 3 1242-6 .188 4.78 Akepa 0 3B None .203 5.16 Akiapolaau 1A 3A 1242-7 .219 5.56 Akikiki 0, 1C, 1 4 1242-8 .250 6.35 Akohekohe 1A 4S 1242-8 .250 6.35 Alaska Marbled Murrelet 3B, 3 4A None .281 7.14 Alder Flycatcher 0, 0A 4AS None .281 7.14 Aleutian Canada Goose 7B 5 1242-10 .313 7.95 Aleutian Tern 2, 1A, 1D 5A None .344 8.73 Allen's Hummingbird X 6 1242-12 .375 9.53 Altamira Oriole 3 7A 1242-14 .438 11.13 American Avocet 4, 4A 7AS 1242-14 .438 11.13 American Bittern M: 7A F: 6 7 1242-16 .500 12.70 American Black Duck 7A 7B None .531 13.49 American -

PUERTO RICO December 5-11, 2020
® field guides BIRDING TOURS WORLDWIDE [email protected] • 800•728•4953 ITINERARY PUERTO RICO December 5-11, 2020 The Puerto Rican Screech-Owl is one of the endemics we’ll see on the island. Unlike other screech-owls, this species does not have ear-tufts. Photograph by guide Tom Johnson. We include here information for those interested in the 2020 Field Guides Puerto Rico tour: ¾ a general introduction to the tour ¾ a description of the birding areas to be visited on the tour ¾ an abbreviated daily itinerary with some indication of the nature of each day’s outings Those who register for the tour will be sent this additional material: ¾ an annotated list of the birds recorded on a previous year’s Field Guides trip to the area, with comments by guide(s) on notable species or sightings (may be downloaded from our web site) ¾ a detailed information bulletin with important logistical information and answers to questions regarding accommodations, air arrangements, clothing, currency, customs and immigration, documents, health precautions, and personal items ¾ a reference list ¾ a Field Guides checklist for preparing for and keeping track of the birds we see on the tour ¾ after the conclusion of the tour, a list of birds seen on the tour Puerto Rico, easternmost and smallest of the Greater Antilles, is an exciting birding destination that harbors seventeen species of birds found nowhere else. Additionally, this US-associated Commonwealth features fine facilities and a good road system, making birding in Puerto Rico—from montane rainforests and elfin woods to mangrove swamps and dry coastal scrub—a comfortable and rewarding experience.