The Digestive Tract As the Origin of Systemic Inflammation Petrus R
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Diagnosis and Treatment of Perianal Crohn Disease: NASPGHAN Clinical Report and Consensus Statement
CLINICAL REPORT Diagnosis and Treatment of Perianal Crohn Disease: NASPGHAN Clinical Report and Consensus Statement ÃEdwin F. de Zoeten, zBrad A. Pasternak, §Peter Mattei, ÃRobert E. Kramer, and yHoward A. Kader ABSTRACT disease. The first description connecting regional enteritis with Inflammatory bowel disease is a chronic inflammatory disorder of the perianal disease was by Bissell et al in 1934 (2), and since that time gastrointestinal tract that includes both Crohn disease (CD) and ulcerative perianal disease has become a recognized entity and an important colitis. Abdominal pain, rectal bleeding, diarrhea, and weight loss consideration in the diagnosis and treatment of CD. Perianal characterize both CD and ulcerative colitis. The incidence of IBD in the Crohn disease (PCD) is defined as inflammation at or near the United States is 70 to 150 cases per 100,000 individuals and, as with other anus, including tags, fissures, fistulae, abscesses, or stenosis. autoimmune diseases, is on the rise. CD can affect any part of the The symptoms of PCD include pain, itching, bleeding, purulent gastrointestinal tract from the mouth to the anus and frequently will include discharge, and incontinence of stool. perianal disease. The first description connecting regional enteritis with perianal disease was by Bissell et al in 1934, and since that time perianal INCIDENCE AND NATURAL HISTORY disease has become a recognized entity and an important consideration in the Limited pediatric data describe the incidence and prevalence diagnosis and treatment of CD. Perianal Crohn disease (PCD) is defined as of PCD. The incidence of PCD in the pediatric age group has been inflammation at or near the anus, including tags, fissures, fistulae, abscesses, estimated to be between 13.6% and 62% (3). -

Descriptive Study Regarding the Etiological Factors Responsible for Secondary Bacterial Peritonitis in Patients Admitted in a Te
International Journal of Health Sciences and Research Vol.10; Issue: 7; July 2020 Website: www.ijhsr.org Original Research Article ISSN: 2249-9571 Descriptive Study Regarding the Etiological Factors Responsible for Secondary Bacterial Peritonitis in Patients Admitted in a Tertiary Care Hospital in Trans Himalayan Region Raj Kumar1, Rahul Gupta2, Anjali Sharma3, Rajesh Chaudhary4 1MS General Surgery, Civil Hospital Baijnath, Himachal Pradesh 2MD Community Medicine, District Programme Officer, Health and Family Welfare, Himachal Pradesh 3Resident Doctor, Department of Microbiology, DRPGMC Kangra at Tanda, Himachal Pradesh 4MS General Surgery, Civil Hospital Nagrota Bagwan, Himachal Pradesh Corresponding Author: Rahul Gupta ABSTRACT Peritonitis is an inflammation of the peritoneum. Primary peritonitis which is spontaneous bacterial peritonitis, Secondary peritonitis due to infection from intraabdominal source or spillage of its contents and Tertiary peritonitis which is recurrent or reactivation of secondary peritonitis. The present study was aimed to determine the etiology of generalized secondary peritonitis among the patients admitted in Department of General Surgery, Dr RPGMC Kangra at Tanda. This descriptive observational study was conducted in the department of surgery Dr. Rajendra Prasad Government Medical College Kangra at Tanda consisting of patients having acute generalised secondary peritonitis presented in emergency department or Surgery outdoor patient department over a period of one year from December 2016 through November 2017. The most common etiology of generalized secondary peritonitis in our patients was peptic ulcer disease (77.13%) followed by perforated appendicitis (9.8%). Etiological factors of secondary generalised peritonitis have a different pattern in different geographical regions. Peptic ulcer disease remains the commonest etiology of secondary peritonitis in India followed by enteric perforation which is in contrast to the western studies where appendicular and colon perforations are more common. -

PERFORATED PEPTIC ULCER. Patient Usually Experiences
Postgrad Med J: first published as 10.1136/pgmj.12.134.470 on 1 December 1936. Downloaded from 470 POST-GRADUATE MEDICAL JOURNAL December, 1936 PERFORATED PEPTIC ULCER. By RONALD W. RAVEN, F.R.C.S. (Assistant Surgeon to T'he French Hospital, Assistant Surgeon to The Gordon Hospital for Rectal Diseases and Swrgical Registrar to The Royal Cancer Hospital.) INTRODUCTION. Peptic ulceration is a crippling disease judged from the stand-point of morbidity, and is also dangerous to life on account of serious complications, such as haemorrhage or perforation which may supervene during the course of the disease. These complications may occur in any patient and there are no criteria which will indicate whether or not an ulcer will bleed or perforate. When the treatment of peptic ulceration is under review it must be remembered that from 20 to 30 per cent. of these ulcers perforate. In a large series of cases I found that the incidence of perforation was 27 per cent. It is thus essential that patients suffering with peptic ulcer should be kept under continuous careful observation. Unfortunately, however, a small percentage of patients give no previous history of the peptic ulcer syndrome and perforation of the ulcer is the first indication of its presence. Recently, when considering the role of surgery in the treatment of chronic peptic ulcer, Joll stated that there has been a rise in the incidence of perforation as a complication of peptic ulcer since medical treatment has become systematized in the treatment of this disease. It must also be remembered that medical treat- Protected by copyright. -
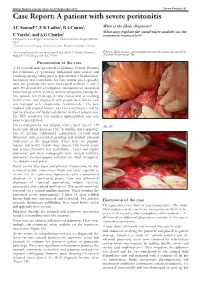
Case Report: a Patient with Severe Peritonitis
Malawi Medical Journal; 25(3): 86-87 September 2013 Severe Peritonitis 86 Case Report: A patient with severe peritonitis J C Samuel1*, E K Ludzu2, B A Cairns1, What is the likely diagnosis? 2 1 What may explain the small white nodules on the C Varela , and A G Charles transverse mesocolon? 1 Department of Surgery, University of North Carolina, Chapel Hill NC USA 2 Department of Surgery, Kamuzu Central Hospital, Lilongwe Malawi Corresponding author: [email protected] 4011 Burnett Womack Figure1. Intraoperative photograph showing the transverse mesolon Bldg CB 7228, Chapel Hill NC 27599 (1a) and the pancreas (1b). Presentation of the case A 42 year-old male presented to Kamuzu Central Hospital for evaluation of worsening abdominal pain, nausea and vomiting starting 3 days prior to presentation. On admission, his history was remarkable for four similar prior episodes over the previous five years that lasted between 3 and 5 days. He denied any constipation, obstipation or associated hematemesis, fevers, chills or urinary symptoms. During the first episode five years ago, he was evaluated at an outlying health centre and diagnosed with peptic ulcer disease and was managed with omeprazole intermittently . His past medical and surgical history was non contributory and he had no allergies and he denied alcohol intake or tobacco use. His HIV serostatus was negative approximately one year prior to presentation. On examination he was afebrile, with a heart rate of 120 (Fig 1B) beats/min, blood pressure 135/78 mmHg and respiratory rate of 22/min. Abdominal examination revealed mild distension with generalized guarding and marked rebound tenderness in the epigastrium. -

Digestive Tract Tuberculosis
World Gastroenterology Organisation Global Guidelines Digestive tract tuberculosis March 2021 WGO Review Team Mohamed Tahiri (Chair, Morocco), K.L. Goh (Co-Chair, Malaysia), Zaigham Abbas (Pakistan), David Epstein (South Africa), Chen Min-Hu (China), Chris Mulder (Netherlands), Amarender Puri (India), Michael Schultz (New Zealand), Anton LeMair (Netherlands) Funding and conflict of interest statement All of the authors have stated that there were no conflicts of interest in relation to their authorship of this paper. Anton LeMair acts as guideline development consultant for WGO. WGO Global Guidelines Digestive tract tuberculosis 2 Contents 1 Introduction .............................................................................................................................. 4 1.1 About WGO cascades ................................................................................................................. 5 1.2 Definitions .................................................................................................................................. 5 1.3 Epidemiology .............................................................................................................................. 6 1.3.1 WHO 2018 global tuberculosis report .............................................................................. 6 1.4 Etiopathogenesis and risk factors .............................................................................................. 7 2 Clinical features ....................................................................................................................... -
A Case Series on Intussusceptions in Infants Presenting with Listlessness
ABDOMINAL CONDITIONS © 2010 SNL All rights reserved A case series on intussusceptions in infants presenting with listlessness Intussusception is characterised by abdominal pain, vomiting and blood in stools. However, in younger infants it may present with non-classical symptoms such as listlessness, pallor, decreased feeding, and being non-specifically unwell. Three cases of intussusception in young infants who presented with being listless and had some or no features to suggest a clinical diagnosis of intussusception are described which are designed to highlight the non-classical features of intussusception likely to be encountered in very young infants. Siba P. Paul1 ntussusception is one of the most tachycardia and prolonged capillary refill MBBS, DCH Icommon surgical emergencies time he was given a fluid bolus of 20mL/kg Paediatric Trust Registrar encountered in infancy and early with 0.9% sodium chloride. He was [email protected] childhood. This is a condition where the intermittently responding to his parents proximal segment of the bowel telescopes but was not irritable. His cardiovascular 1 David C. A. Candy into the distal segment causing and respiratory examination was MBBS, MSc, MD, FRCP, FRCPCH, FCU obstruction1. The classic triad of symptoms otherwise normal. Consultant Paediatric Gastroenterologist consists of abdominal pain, vomiting and He was distressed by an abdominal blood in stools1. It is often seen in children Nikila Pandya2 examination and was found to be drawing aged four months to two years, with a peak up his legs and crying while being MD, DCH, FRCPCH incidence during four to nine months of Consultant Paediatrician examined. The provisional diagnosis at age2. -

A Challenging Case of Recurrent Eosinophilic Peritonitis
Open Access Case Report DOI: 10.7759/cureus.9422 A Challenging Case of Recurrent Eosinophilic Peritonitis Myra Nasir 1 , Jasmin Hundal 1 , Arish Noor 1 , Juan Jose Chango Azanza 1 , Jaimy Villavicencio 1 1. Internal Medicine, University of Connecticut, Farmington, USA Corresponding author: Myra Nasir, [email protected] Abstract Eosinophilic peritonitis is a rare presentation of eosinophilic gastroenteritis and is characterized by eosinophil-rich inflammation in any part of the gastrointestinal tract in the absence of secondary causes of eosinophilia. We report a case of a 48-year-old female who had recurrent hospital admissions due to abdominal pain and distension secondary to relapsing eosinophilic peritonitis. Categories: Allergy/Immunology, Gastroenterology Keywords: idiopathic eosinophilic peritonitis, eosinophilic gastroenteritis, ascites Introduction Eosinophilic peritonitis (EP) is a rare presentation of eosinophilic gastroenteritis (EGE) [1]. Patients often present with abdominal distension, which can be accompanied by nausea, vomiting, diarrhea, and abdominal pain. The pathogenesis is poorly understood. We report the case of a 48-year-old female who had recurrent admissions for abdominal pain and distension and was found to have eosinophilic cholecystitis and EP. Case Presentation A 48-year-old female with a past medical history significant for asthma and bronchitis presented to the hospital in October 2018 with worsening abdominal pain associated with abdominal distension evolving over three weeks and diarrhea for three days. One month prior to this, she had undergone cholecystectomy, with tissue biopsy revealing eosinophilic cholecystitis (Figure 1). Her medications included furosemide 20 mg and pantoprazole 40 mg daily. She denied using any over-the-counter or herbal medications. Physical examination revealed a distended abdomen, diffusely tender to palpation. -

Abdominal Pain
10 Abdominal Pain Adrian Miranda Acute abdominal pain is usually a self-limiting, benign condition that irritation, and lateralizes to one of four quadrants. Because of the is commonly caused by gastroenteritis, constipation, or a viral illness. relative localization of the noxious stimulation to the underlying The challenge is to identify children who require immediate evaluation peritoneum and the more anatomically specific and unilateral inner- for potentially life-threatening conditions. Chronic abdominal pain is vation (peripheral-nonautonomic nerves) of the peritoneum, it is also a common complaint in pediatric practices, as it comprises 2-4% usually easier to identify the precise anatomic location that is produc- of pediatric visits. At least 20% of children seek attention for chronic ing parietal pain (Fig. 10.2). abdominal pain by the age of 15 years. Up to 28% of children complain of abdominal pain at least once per week and only 2% seek medical ACUTE ABDOMINAL PAIN attention. The primary care physician, pediatrician, emergency physi- cian, and surgeon must be able to distinguish serious and potentially The clinician evaluating the child with abdominal pain of acute onset life-threatening diseases from more benign problems (Table 10.1). must decide quickly whether the child has a “surgical abdomen” (a Abdominal pain may be a single acute event (Tables 10.2 and 10.3), a serious medical problem necessitating treatment and admission to the recurring acute problem (as in abdominal migraine), or a chronic hospital) or a process that can be managed on an outpatient basis. problem (Table 10.4). The differential diagnosis is lengthy, differs from Even though surgical diagnoses are fewer than 10% of all causes of that in adults, and varies by age group. -

Emergency Surgery 2 Perforated Peptic Ulcer
Series Emergency surgery 2 Perforated peptic ulcer Kjetil Søreide, Kenneth Thorsen, Ewen M Harrison, Juliane Bingener, Morten H Møller, Michael Ohene-Yeboah, Jon Arne Søreide Lancet 2015; 386: 1288–98 Perforated peptic ulcer is a common emergency condition worldwide, with associated mortality rates of up to 30%. See Editorial page 1212 A scarcity of high-quality studies about the condition limits the knowledge base for clinical decision making, but a few This is the second in a Series of published randomised trials are available. Although Helicobacter pylori and use of non-steroidal anti-infl ammatory three papers about emergency drugs are common causes, demographic diff erences in age, sex, perforation location, and underlying causes exist surgery between countries, and mortality rates also vary. Clinical prediction rules are used, but accuracy varies with study Department of population. Early surgery, either by laparoscopic or open repair, and proper sepsis management are essential for good Gastrointestinal Surgery, Stavanger University Hospital, outcome. Selected patients can be managed non-operatively or with novel endoscopic approaches, but validation of Stavanger, Norway such methods in trials is needed. Quality of care, sepsis care bundles, and postoperative monitoring need further (Prof K Søreide MD, assessment. Adequate trials with low risk of bias are urgently needed to provide better evidence. We summarise the K Thorsen MD, evidence for perforated peptic ulcer management and identify directions for future clinical research. Prof J A Søreide MD); Department of Clinical Medicine, University of Introduction important role in diagnosis, as does early resuscitation, Bergen, Bergen, Norway Perforated peptic ulcer is a surgical emergency and is including administration of antibiotics. -

Or Ascending Cholangitis) Is a Clinical Syndrome That Is Classically Characterized by the Triad Of
ACUTE ASCENDING CHOLANGITIS Introduction Acute cholangitis (or ascending cholangitis) is a clinical syndrome that is classically characterized by the triad of: ● Fever ● Abdominal pain ● Jaundice The condition occurs as a result of obstruction/ stasis and subsequent infection within the biliary tract. In the absence of appropriate treatment the condition has significant potential for mortality and morbidity. It is a medical emergency that requires prompt antibiotic treatment and urgent relief of biliary obstruction (usually by endoscopic retrograde cholangiopancreatography ERCP). History Jean M. Charcot was the first to describe this illness in 1877. He described a triad of fever, jaundice, and right upper quadrant pain. Epidemiology Acute cholangitis is predominantly seen in adults, most commonly around 40-70 years of age. Physiology Normal barrier mechanisms to cholangitis infection include ● The sphincter of Oddi, which normally forms an effective mechanical barrier to duodenal reflux and ascending bacterial infection. ● Continuous flushing action of bile plus the bacteriostatic activity of bile salts which help maintain bile sterility. ● Secretory IgA and biliary mucous probably also function as anti-adherence factors, preventing bacterial colonization Pathology Organisms: Ascending cholangitis is usually associated with Gram-negative or anaerobic sepsis. ● Aerobic organisms include Escherichia coli and Klebsiella and Enterococcus species. ● The most common anaerobic organism is Bacteroides fragilis. Clostridia may also cause serious infection. Causes: Ascending cholangitis essentially occurs as consequence of obstruction of the biliary tract. Stasis leads to secondary bacterial infection; the organisms typically ascending from the duodenum. Biliary obstruction raises intrabiliary pressure and leads to increased permeability of bile ductules, permitting translocation of bacteria and toxins from the portal circulation into the biliary tract. -

Fact Sheet: News from the IBD Help Center: Intestinal Complications
Fact Sheet News from the IBD Help Center INTESTINAL COMPLICATIONS The complications of Crohn’s disease and ulcerative colitis are generally classified as either local or systemic. The term “local” refers to complications involving the intestinal tract itself, while the term “systemic” (or extraintestinal) refers to complications that involve other organs or that affect the patient as a whole. Intestinal complications tend to occur when the intestinal inflammation: • is severe • extends beyond the inner lining (mucosa) of the intestines • is widespread • is chronic (of long duration) Some intestinal complications occur in both ulcerative colitis and Crohn’s disease, although they may occur more commonly in one than in the other. Not everyone will experience these complications. However, early recognition and prompt treatment are key. If you notice a change in your symptoms, be sure to contact your doctor immediately. Ulcerative Colitis-Local Complications Perforation (rupture) of the bowel. Intestinal perforation occurs when chronic inflammation and ulceration of the intestine weakens the wall to such an extent that a hole develops in the intestinal wall. This perforation is potentially life- threatening because the contents of the intestine, which contain a large number of bacteria, can spill into the abdomen and cause a serious infection called peritonitis. In colitis, this complication is generally linked with toxic megacolon (see below). In Crohn’s disease, it may occur as a result of an abscess or fistula. Fulminant colitis. This complication, which affects less than 10% of people with colitis, involves damage to the entire thickness of the intestinal wall. When severe inflammation causes the colon to become extremely dilated and swollen, a condition called ileus may develop. -

Septic Peritonitis
3 CE CE Article CREDITS Septic Peritonitis William T. N. Culp, VMD, DACVS University of California-Davis David E. Holt, BVSc, DACVS University of Pennsylvania Abstract: Bacterial septic peritonitis is a serious condition that requires immediate treatment. The pathogenesis is complex, and the list of diagnostic differentials is extensive. The keys to successful treatment are early recognition of the condition and elimination of the causative organism. Multiple options for draining the peritoneal cavity exist, and further studies are neces- sary to establish specific, evidence-based guidelines. The prognosis is generally guarded in dogs and cats. Much depends on whether the patient develops concurrent sepsis, systemic inflammatory response syndrome, or multiple organ dysfunction syndrome. eptic peritonitis is a life-threatening condition that Neutrophils, Macrophages, and Mast Cells occurs secondary to many intraabdominal diseases in Normally, the peritoneal cavity contains a diverse array of Sdogs and cats. This article reviews bacterial peritonitis cells capable of reacting to antigens.10,11 When a peritoneal and sepsis and describes treatment options and prognosis. injury occurs, vasoactive substances (e.g., histamine) are released. Histamine from degranulating resident peritoneal Anatomy and Intrinsic Defense Systems of the mast cells stimulates vasodilation and exudation of fluid con- Peritoneal Cavity taining complement and opsonins that are capable of coat- Mesothelium ing bacteria, promoting phagocytosis and encouraging an The peritoneal cavity is lined with a serous layer of meso- influx of neutrophils and macrophages into the peritoneal thelial cells.1–3 After peritoneal injury, regeneration begins cavity.7,10,11 Peritoneal mast cells, neutrophils, macrophages, with an influx of round cells that eventually transform into and lymphocytes interact to promote cytokine expression, mesothelial cells.