Use of Plant-Associated Bacillus Strains As Biofertilizers and Biocontrol Agents in Agriculture
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Effects of Biofertilizers Combined with Different Soil Amendments on Potted Rice Plants
RESEARCH 157 EFFECTS OF BIOFERTILIZERS COMBINED WITH DIFFERENT SOIL AMENDMENTS ON POTTED RICE PLANTS Arshad Javaid1* ABSTRACT This pot study investigated the effect of the combined application of two commercial biofertilizers viz. Biopower and EM (Effective Microorganisms) on rice (Oryza sativa L.) growth and yield in soils amended with farmyard manure, green manure, and NPK fertilizers. Biopower is a product of the Nuclear Institute for Biotechnology and Genetic Engineering (NIBGE), Pakistan, which contains species of associative and endophytic diazotrophs. EM (effective microorganisms), a product developed by Japanese scientists, consists of co-existing beneficial microorganisms, mainly species of photosynthetic and lactic acid bacteria, as well as yeast. Applying Biopower adversely affected plant growth and yield in NPK fertilizer amendment. Conversely, this biofertilizer markedly enhanced plant growth and yield in green manure amended soil while its effect was not significant in farmyard manure amendment. In green manure amendment, applying EM enhanced grain yield by 46%. Co-inoculation of Biopower and EM evidently improved root and shoot growth in farmyard manure amended soil. This study concludes that the two biofertilizers clearly enhanced shoot biomass and grain yield in green manure amended soils. Key words: Associated N2-fixers, Biopower, effective microorganisms, rice, soil amendments. INTRODUCTION several bacteria may be isolated from sterilized surface roots of flooded rice plants, suggesting endophytic Rice (Oryza sativa L.) is probably the most important colonization (Raimam et al., 2007). The most likely cereal in the world and serves as food for about 50% of candidates for biological N fixation in rice are species the world’s population (Ladha et al., 1997). -

Bio-Fertilizers- Power of Beneficial Microorganisms in Soils
DOI: 10.26717/BJSTR.2018.04.001076 Nur Okur. Biomed J Sci & Tech Res ISSN: 2574-1241 Mini review Open Access A Review: Bio-Fertilizers- Power of Beneficial Microorganisms in Soils Nur Okur* Department of Soil Science and Plant Nutrition, Ege University, Turkey Received: May 01, 2018; Published: May 16, 2018 *Corresponding author: Nur Okur, Departmentof Soil Science and Plant Nutrition, Ege University, Turkey Abstract uptake of nutrients by their interactions in the rhizosphere when applied through seed or soil. They accelerate certain microbial processes Bio-fertilizers are defined as preparations containing living cells or latent cells of efficient strains of microorganisms that help crop plants’ important components of integrated nutrient management, as they are cost effective and renewable source of plant nutrients to supplement thein the chemical soil which fertilizers augment for sustainablethe extent of agriculture. availability of nutrients in a form easily assimilated by plants. The use of bio-fertilizers is one of the Keywords: Bio-fertilizer; Sustainable agriculture; Liquid bio-fertilizer Abbreviations: PGPB: Plant Growth Promoting Bio-Fertilizer; KSB: Potassium Solubilizing Bio-Fertilizer; KMB: Potassium Mobilizing Bio- Fertilizer; SOB: Sulfur Oxidizing Bio-Fertilizer; NFB: Nitrogen Fixing Bio-Fertilizers Introduction Some strategies increasing the quality of the soil are needed to ensure sustainability in soil fertility. Increasing the population of a) more cost-effective than chemical fertilizers, availability of nitrogen -

Assessment of Multifunctional Biofertilizers on Tomato Plants Cultivated Under a Fertigation System ABSTRACT Malaysian Nuclear A
Assessment of multifunctional biofertilizers on tomato plants cultivated under a fertigation system Phua, C.K.H., Abdul Wahid, A.N. and Abdul Rahim, K. Malaysian Nuclear Agency (Nuclear Malaysia) Ministry of Science, Technology and Innovation, Malaysia (MOSTI) E-mail: [email protected] ABSTRACT Malaysian Nuclear Agency (Nuclear Malaysia) has developed a series of multifunctional bioorganic fertilizers, namely, MULTIFUNCTIONAL BIOFERT PG & PA and MF- BIOPELLET, in an effort to reduce dependency on chemical fertilizer for crop production. These products contain indigenous microorganisms that have desired characteristics, which include plant growth promoting, phosphate solubilising, antagonistic towards bacterial wilt disease and enhancing N2-fixing activity. These products were formulated as liquid inoculants, and introduced into a fertigation system in an effort to reduce usage of chemical fertilizers. A greenhouse trial was conducted to evaluate the effectiveness of multifunctional biofertilizers on tomato plants grown under a fertigation system. Multifunctional biofertilizer products were applied singly and in combination with different rates of NPK in the fertigation system. Fresh and dry weights of tomato plants were determined. Application of multifunctional biofertilizer combined with 20 g NPK resulted in significantly higher fresh and dry weights as compared to other treatments. ABSTRAK Agensi Nuklear Malaysia (Nuklear Malaysia) telah membangunkan satu siri baja bioorganic pelbagai fungsi, iaitu MULTIFUNCTIONAL BIOFERT PG & PA and MF-BIOPELLET, dalam usaha mengurangkan pergantungan terhadap baja kimia dalam penghasilan tanaman. Produk ini mengandungi mikroorganisma setempat yang menpunyai ciri yang dikehendaki seperti penggalak pertumbuhan, pengurai fosfat, antagonis terhadap penyakit layu bakteria dan menggalak aktiviti pengikat N2. Produk ini difomulasi dalam bentuk cecair dan diperkenalkan ke dalam sistem fertigasi untuk mengurangkan penggunaan baja kimia. -

Effect of Chemical, Organic and Bio Fertilizers on Photosynthetic Pigments, Carbohydrates and Minerals of Wheat (Triticum Aestivum
Int. J. Adv. Res. Biol. Sci. (2016). 3(2): 296-310 Research Article SOI: http://s-o-i.org/1.15/ijarbs-2016-3-2-40 Effect of Chemical, Organic and Bio Fertilizers on photosynthetic pigments, carbohydrates and minerals of Wheat (Triticum aestivum. L) Irrigated with Sea Water Amany S. Al-Erwy*, Abdulmoneam Al-Toukhy and Sameera O. Bafeel Dept. Biological Sciences, Faculty of Sciences King Abdul Aziz Univ, KSA *Corresponding author: [email protected] Abstract The present study was conducted to investigate the effect of chemical, organic and bio-fertilizers on photosynthetic pigments, soluble sugars, non-soluble sugars, total carbohydrates and mineral elements in wheat (Triticum aestivum L.) plants grown under different concentrations of sea water (0%, 20% and 40%). Chemical fertilizer was used at concentrations of 0, 250 and 500 kg/ha; Rhizobium and Azotobacter were used as Biofertilizers; and Humic acid in concentrations of (0, 5 and 10 kg/ha) was used as organic fertilizer. The obtained results showed that photosynthetic pigments, carbohydrates and nutrient elements were markedly reduced at the high levels of sea water particularly 40% ratio. While, fertilizer treatments had an observed promotion effects on those constituents, particularly Bio and organic fertilizers that were more effective than chemical fertilizers even at high concentrations of sea water. This may be because of the potential effect of organic and bio- fertilizers on providing the nutrient elements needed by plants besides some other beneficial compounds that help plants to withstand high salt stress conditions. Keywords: Sea water, fertilizers, wheat, pigments, carbohydrates, minerals. Introduction Wheat (Triticum aestivum, L.) is one of the most Saudi Arabia as well as other arid and semi-arid important crops in most countries of the world regions all over the world (Almaghrabi, 2012). -

Bio-Organic Fertilizers Stimulate Indigenous Soil Pseudomonas Populations to Enhance Plant Disease Suppression
Tao et al. Microbiome (2020) 8:137 https://doi.org/10.1186/s40168-020-00892-z RESEARCH Open Access Bio-organic fertilizers stimulate indigenous soil Pseudomonas populations to enhance plant disease suppression Chengyuan Tao1,2†, Rong Li1,2†, Wu Xiong3, Zongzhuan Shen1,2, Shanshan Liu1,2, Beibei Wang4, Yunze Ruan4, Stefan Geisen5,6, Qirong Shen1,2,7* and George A. Kowalchuk3 Abstract Background: Plant diseases caused by fungal pathogen result in a substantial economic impact on the global food and fruit industry. Application of organic fertilizers supplemented with biocontrol microorganisms (i.e. bioorganic fertilizers) has been shown to improve resistance against plant pathogens at least in part due to impacts on the structure and function of the resident soil microbiome. However, it remains unclear whether such improvements are driven by the specific action of microbial inoculants, microbial populations naturally resident to the organic fertilizer or the physical-chemical properties of the compost substrate. The aim of this study was to seek the ecological mechanisms involved in the disease suppressive activity of bio-organic fertilizers. Results: To disentangle the mechanism of bio-organic fertilizer action, we conducted an experiment tracking Fusarium wilt disease of banana and changes in soil microbial communities over three growth seasons in response to the following four treatments: bio-organic fertilizer (containing Bacillus amyloliquefaciens W19), organic fertilizer, sterilized organic fertilizer and sterilized organic fertilizer supplemented with B. amyloliquefaciens W19. We found that sterilized bioorganic fertilizer to which Bacillus was re-inoculated provided a similar degree of disease suppression as the non-sterilized bioorganic fertilizer across cropping seasons. -

Multifunctional Liquid Biofertilizer As An
MULTIFUNCTIONAL LIQUID BIOFERTILIZER AS AN INNOVATIVE AGRONOMIC INPUT FOR MODERN AGRICULTURE BIOBAJA CECAIR MULTIFUNGSISEBAGAI INPUTAGRONOMIINOVATIF UNTUK PERTANIAN MODEN Phua Chop Kwai Hoe and Khairuddin Abdul Rahim Agensi Nuklear Malaysia (Nuklear Malaysia) Kementerian Sains, Teknologi dan Inovasi (MOSTT), Bangi, 43000 KAJANG, Selangor E-mail: [email protected] Abstract Liquid biofertilizer is increasingly available in the market as one of the alternatives to chemical and organic fertilizers as well as solid substrate-based biofertilizers. One of the benefits from biofertilizer is the contribution from population of microorganisms available. These microorganisms may enhance the plant growth and create healthy rhizosphere. The advantage of a liquid biofertilizer is that no solid carrier is needed. These products are also developed for potential application in modem agriculture such as soilless farming systems, viz. fertigation and hydroponics. Traditionally, liquid biofertilizer is produced from fermentation of effective microorganisms which was recommended to be used within three months. Therefore, the development of low-cost and long shelf-life liquid biofertilizers was conducted at Malaysian Nuclear Agency (Nuclear Malaysia). Three biofertilizer inoculums (phosphate solubilising bacteria and plant growth promoting bacteria) were developed into four formulations of liquid biofertilizers. The liquid biofertilizers were kept at low temperatures (9 + 2 °C) and room temperatures (28 ± 2°C) for shelf-life study. Nutrient broth liquid biofertilizer kept at low temperatures showed significantly high survival rates after storage for six months as compared to other formulations and treatments. Keywords: Liquid biofertilizer, phosphate solubilising bacteria, plant growth promoting bacteria, shelf life; soilless system Abstrak Permintaan biobaja cecair di pasaran semakin meningkat, di mana ia adalah altematif kepada baja kimia, baja organik dan biobaja substrat pepejal. -

Chemical and Biological Enhancement Effects of Biochar on Wheat Growth and Yield Under Arid Field Conditions
sustainability Article Chemical and Biological Enhancement Effects of Biochar on Wheat Growth and Yield under Arid Field Conditions Zarmeena Khan 1, Muhammad Habib ur Rahman 1,2,* , Ghulam Haider 1,3, Rabia Amir 3, Rao Muhammad Ikram 1, Shakeel Ahmad 4, Hannah Kate Schofield 5 , Bilal Riaz 1, Rashid Iqbal 6 , Shah Fahad 7,* , Rahul Datta 8,* , Alaa Baazeem 9 , Ayman EL Sabagh 10 and Subhan Danish 11,* 1 Department of Agronomy, MNS-University of Agriculture, Multan 60000, Pakistan; [email protected] (Z.K.); [email protected] (G.H.); [email protected] (R.M.I.); [email protected] (B.R.) 2 Crop Science, Institute of Crop Science and Resource Conservation (INRES), University of Bonn, 53115 Bonn, Germany 3 Department of Plant Biotechnology, Atta-Ur-Rahman School of Applied Biosciences, National University of Sciences and Technology (NUST), Islamabad 44000, Pakistan; [email protected] 4 Department of Soil Science, MNS-University of Agriculture, Multan 60000, Pakistan; [email protected] 5 Biogeochemistry Research Centre, School of Geography, Earth and Environmental Science, University of Plymouth, Plymouth PL4 8AA, UK; kate.schofi[email protected] 6 Department of Agronomy, Faculty of Agriculture & Environment, The Islamia University of Bahawalpur, Bahawalpur, Punjab 63100, Pakistan; [email protected] 7 Citation: Khan, Z.; Rahman, M.H.u.; Department of Agronomy, The University of Haripur, Haripur 22620, Pakistan 8 Haider, G.; Amir, R.; Ikram, R.M.; Department of Geology and Pedology, Faculty of Forestry and Wood Technology, Mendel University in Brno, Zemedelska1, 61300 Brno, Czech Republic Ahmad, S.; Schofield, H.K.; Riaz, B.; 9 Department of Biology, College of Science, Taif University, P.O. -

Review Article Biofertilizer: an Alternative of Synthetic Fertilizers
1 Plant Archives Vol. 20, Supplement 2, 2020 pp. 1374-1379 e-ISSN:2581-6063 (online), ISSN:0972-5210 REVIEW ARTICLE BIOFERTILIZER: AN ALTERNATIVE OF SYNTHETIC FERTILIZERS Swapnil Rai and Nidhi Shukla Amity Institute of Environmental Science, Amity University Madhya Pradesh, Gwalior, India - 474005 [email protected], [email protected] Abstract India is one of the nations that have large geographical area under agriculture since time immemorial. Most of the population of India is dependent upon the agriculture and are indiscriminately using chemical fertilizer in agriculture without following any guidelines. These chemicals are causing soil pollution and water pollution which reduces the crop production and are also affecting the living being including humans. When the synthetic fertilizers applied in excess, get accumulated in soil and plant or washed away with the water during monsoon season results the contamination of water bodies. Now days, biofertilizers have identified as an alternative of chemical fertilizers as they don’t affect the environment and human health. These are eco-friendly substances, causing no effects on soil properties and economic. The living cells of biofertilizers convert naturally occurring minerals in to usable nutrients for plants and enhance the crop production. This paper deals with significant biological agents which have potential of biofertilizer. In India there is a need of innovation; research and public awareness for biofertilizer which is also important for sustainable agriculture. Keywords : Biofertilizer, eco-friendly, pollution, health hazard Introduction water bodies, also affect fishes (Palanichamy, 1985) and In India, due to growing human population it is other organisms. The extensive applications of synthetic fertilizers from last several years have not only caused soil necessary to increase crop production and land productivity to fulfill their food requirements. -
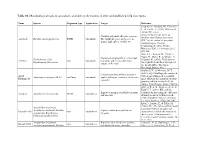
Table S1. Microbiological Tools in Agriculture, Available on the Markets in 2021 with Published Field Test Reports
Table S1. Microbiological tools in agriculture, available on the markets in 2021 with published field test reports. Name Species Organism type Application Target Reference Ladurner, E., Benuzzi, M., Fiorentini, F., & Lucchi, A. (2012). Efficacy of Amylo-X®, a new fungicide/bactericide based on Controls gray mold (Botrytis cinerea), Bacillus amyloliquefaciens strain Amylo-X Bacillus amyloliquefaciens PGPR biocontrol Fire blight (Erwinia amylovora) in D747 for the control of microbial grapes, apple,pPear, strawberry plant pathogens. Giornate Fitopatologiche 2012, Milano Marittima (RA), 13-16 marzo 2012, 229-236. Woo, S. L., Ruocco, M., Vinale, F., Nigro, M., Marra, R., Lombardi, N., ... Control of damping-off, seed and root, Trichoderma viride, & Lorito, M. (2014). Trichoderma- ANOKA mix biocontrol nematode wilt in vegetable, tuber, Pseudomonas fluorescens based products and their widespread cotton, chilli crops use in agriculture. The Open Mycology Journal, 8(1). Shishkoff, N., & McGrath, M. T. (2002). AQ10 biofungicide combined Controls powdery mildews in grapes, AQ10 with chemical fungicides or AddQ Ampelomyces quisqalis M-10 soil fungi biocontrol apples, aubergine, tomatoes, strawberry, Biofungicide spray adjuvant for control of cucurbit cucumber powdery mildew in detached leaf culture. Plant Disease, 86(8), 915-918. Alafeea, R. A. A., Alamery, A. A., & Kalaf, I. T. (2019). Effect of bio Improves nitrogen availability in maize fertilizers on increasing the efficiency Azotovit Azotobacter chroococcum PGPR biofertilizer and broccoli of using chemical fertilizers on the yield component of maize (Zea mays L.). Plant Archives, 19(2), 303-306. Katai, J., Sandor, Z., & Tallai, M. (2008). The effect of an artificial and a Azospirillum brasilense, bacterium fertilizer on some soil Azotobacter vinelandii, Bacillus Improves nutrient acquisition on BactoFil A10 mix biofertilizer characteristics and on the biomass of megaterium, B. -

Plant and Soil Characteristics Affected by Biofertilizers from Rocks and Organic Matter Inoculated with Diazotrophic Bacteria and Fungi That Produce Chitosan
Journal of Soil Science and Plant Nutrition, 2013, 13(3), 592-603 Plant and soil characteristics affected by biofertilizers from rocks and organic matter inoculated with diazotrophic bacteria and fungi that produce chitosan L.R. Berger, N.P. Stamford, C.E.R.S. Santos, A.D.S. Freitas, L.O. Franco, T.C.M. Stamford. Department of Agronomy, University Federal Rural of Pernambuco, 52171-900, Recife, Pernambuco, Brazil. *Corresponding author: [email protected] Abstract The aim of this study was to evaluate the effectiveness of a mixed biofertilizer with phosphate and potash rocks (PK biofertilizer) combined with an earthworm compound inoculated with free living diazotrophic bacteria and Cunninghamella elegans, fungi that produces chitosan, on cowpea nodulation, biomass yield and nutrient uptake. The effects of some chemical attributes from an acidic soil of the Brazilian Northeast were also studied. The treatments were as follows: a) biofertilizer enriched in N by free living diazotrophic bacteria(NPKB), applying crustaceous chitosan (ChCru) at a rate 2 mg mL-1; b) NPKB and ChCru at a rate 4 mg mL-1; c) NPKB and ChCru at a rate 6 mg mL-1; d) NPKB and fungi chitosan (ChFu, 2 mg mL-1); e) NPKB+C. elegans (NPKP); f) NPKB without chitosan; g) mineral fertilizers (NPKF); and h) control without NPK fertilizer and chitosan. Biofertilizer treatments increased cowpea nodules biomass, shoot biomass, and total N, P, and K in the shoots. The largest increase was obtained with ChCru, and the highest rate was obtained with NPKP. Furthermore, biofertilizers reduced soil pH and increased the total N and available P and K. -

Effectiveness of Biofertilizer Enriched in N by Beijerinckia Indica On
Journal of Soil Science and Plant Nutrition, 2017, 17 (4) 1040-1057 RESEARCH ARTICLE Effectiveness of biofertilizer enriched in N by Beijerinckia indica on sugarcane grown on an Ultisol and the interactive effects between biofertilizer and sugarcane filter cake Fernando Luiz Nunes Oliveira, Wagner Silva Oliveira*, Newton Pereira Stamford, Emmanuella Vila Nova Silva, Carolina Etienne Rosália e Silva Santos and Ana Dolores Santiago Freitas Department of Agronomy, University Federal Rural of Pernambuco, Av. Dom Manoel de Medeiros, s/n°, 52171- 900, Recife, Pernambuco, Brazil. *Corresponding author: [email protected] Abstract To improve yield and nutrient absorption, the addition of fertilizers to provide nutrients in adequate quantities is recommended. An alternative substitution for soluble fertilizer is the use of Acidithiobacillus bacteria to pro- duce rock biofertilizer that is then mixed with organic matter inoculated with diazotrophic bacteria (Beijerinckia indica) for N enrichment. This study evaluates the effectiveness of biofertilizer on nutrient uptake and its inter- action with sugarcane filter cake in field-grown sugarcane on an Ultisol in the Brazilian rainforest region. The experiment used two NPK fertilizer treatments (biofertilizer and soluble fertilizer) applied at three rates (50, 100 and 150% of the recommended rate [RR]) and a control treatment of earthworm compost (20 t ha-1). The fertil- izer treatments were applied with and without filter cake, in four replicates. Total C and N, available P and K, and exchangeable Ca and Mg in plants (upper shoots, leaves and stems) as well as soil samples were analyzed. The biofertilizer showed similar responses as did the soluble fertilizer in nutrient uptake in the different parts of sugarcane and in the soil samples. -

The Combined Use of Chemical and Organic Fertilizers And/Or Biofertilizer for Crop Growth and Soil Fertility
THE COMBINED USE OF CHEMICAL AND ORGANIC FERTILIZERS AND/OR BIOFERTILIZER FOR CROP GROWTH AND SOIL FERTILITY Jen-Hshuan Chen Department of Soil and Environmental Sciences, National Chung Hsing University 250 Kuo-Kuang Road, Taichung. Taiwan ROC ABSTRACT Plant nutrients are essential for the production of crops and healthy food for the world’s expanding population. Plant nutrients are therefore a vital component of sustainable agriculture. Increased crop production largely relies on the type of fertilizers used to supplement essential nutrients for plants. The nature and the characteristics of nutrient release of chemical, organic and biofertilizers are different, and each type of fertilizer has its advantages and disadvantages with regard to crop growth and soil fertility. The sound management of fertilization must attempt to ensure both an enhanced and safeguarded environment; therefore, a balanced fertilization strategy that combines the use of chemical, organic or biofertilizers must be developed and evaluated. Key words: plant nutrients, sustainable agriculture, balanced fertilization strategy, supplement essential nutrient, biofertilizer INTRODUCTION release of organic material from the root and the subsequent effect of increased microbial For optimum plant growth, nutrients must be activity on nutrient cycling and plant growth. available in sufficient and balanced quantities. In the rhizosphere the quantities and the types Soils contain natural reserves of plant of substrates are different from those in the nutrients, but these reserves are largely in bulk soil and this leads to colonization by forms unavailable to plants, and only a minor different populations of bacteria, fungi, portion is released each year through biological protozoa and nematodes. Other physicochemical activity or chemical processes.