Recommended INN: List 63
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

In Vivo Imaging of Tgfβ Signalling Components Using Positron
REVIEWS Drug Discovery Today Volume 24, Number 12 December 2019 Reviews KEYNOTE REVIEW In vivo imaging of TGFb signalling components using positron emission tomography 1 1 2 Lonneke Rotteveel Lonneke Rotteveel , Alex J. Poot , Harm Jan Bogaard , received her MSc in drug 3 1 discovery and safety at the Peter ten Dijke , Adriaan A. Lammertsma and VU University in 1 Amsterdam. She is Albert D. Windhorst currently finishing her PhD at the VU University 1 Department of Radiology and Nuclear Medicine, Amsterdam UMC, location VUmc, Amsterdam, The Netherlands Medical Center (VUmc) 2 under the supervision of A. Pulmonary Medicine, Institute for Cardiovascular Research, Amsterdam UMC, location VUmc, Amsterdam, The Netherlands D. Windhorst and Adriaan A. Lammertsma. Her 3 research interest is on the development of positron Department of Cell and Chemical Biology, Oncode Institute, Leiden University Medical Center, Leiden, The emission tomography (PET) tracers that target Netherlands selectively the activin receptor-like kinase 5 in vitro and in vivo. Alex J. Poot obtained his The transforming growth factor b (TGFb) family of cytokines achieves PhD in medicinal chemistry homeostasis through a careful balance and crosstalk with complex from Utrecht University. As postdoctoral researcher at signalling pathways. Inappropriate activation or inhibition of this pathway the VUmc, Amsterdam, he and mutations in its components are related to diseases such as cancer, developed radiolabelled anticancer drugs for PET vascular diseases, and developmental disorders. Quantitative imaging of imaging. In 2014, he accepted a research expression levels of key regulators within this pathway using positron fellowship from Memorial Sloan Kettering Cancer 13 emission tomography (PET) can provide insights into the role of this Center, New York to develop C-labelled probes for tumour metabolism imaging with magnetic resonance in vivo pathway , providing information on underlying pathophysiological imaging (MRI). -

Biological Therapies for Atopic Dermatitis: an Update (Review)
EXPERIMENTAL AND THERAPEUTIC MEDICINE 17: 1061-1067, 2019 Biological therapies for atopic dermatitis: An update (Review) DIANA DELEANU1-3 and IRENA NEDELEA1,2 1Allergology and Immunology Discipline, ‘Iuliu Hatieganu’ University of Medicine and Pharmacy, 400058 Cluj-Napoca; Departments of 2Allergy and 3Internal Medicine, ‘Professor Doctor Octavian Fodor’ Regional Institute of Gastroenterology and Hepatology, 400162 Cluj-Napoca, Romania Received July 6, 2018; Accepted August 22, 2018 DOI: 10.3892/etm.2018.6989 Abstract. Severe atopic dermatitis, which affects both adults in low-income countries (3). Furthermore, the past decades and children, is a debilitating disorder with a significant decline brought a 2-3-fold increase in prevalence in industrialized of patients' quality of life. Although aetiopathogenic factors countries (3). Generally AD onset is in early childhood, as are currently a topic of study and interpretation, the main one of the first steps of the ‘atopic march’, which describes the features of atopic eczema are skin barrier disturbance and natural history of atopic manifestations, and it is character- immune dysregulation. Severe refractory disease that fails to ized by xerotic skin and acute flare-ups of intensely pruritic improve with conventional therapy may benefit from biologic eczematous lesions (4). Recent studies recognize a predilection therapy. Progress in understanding immunopathology of atopic of AD for persistence in adulthood, with a lifetime prevalence dermatitis have allowed identification of therapeutic molecular accounting for 34.1% (5). Early onset, allergic rhinitis and targets in the field of biological therapy. We reviewed the hand eczema in childhood are high-risk factors for persistent different biological treatments with a focus on novel targeted AD (5). -

New Treatments for Atopic Dermatitis Targeting Skin Barrier Repair Via the Regulation of FLG Expression
Journal of Clinical Medicine Review New Treatments for Atopic Dermatitis Targeting Skin Barrier Repair via the Regulation of FLG Expression Anna D˛ebi´nska 1st Department and Clinic of Paediatrics, Allergology and Cardiology, Wroclaw Medical University, Chałubi´nskiego2a, 50-368 Wrocław, Poland; [email protected]; Tel.: +48-510-066-478 or +71-770-30-91; Fax: +48-713-281-206 Abstract: Atopic dermatitis (AD) is one of the most common chronic, inflammatory skin disorders with a complex etiology and a broad spectrum of clinical phenotypes. Despite its high prevalence and effect on the quality of life, safe and effective systemic therapies approved for long-term management of AD are limited. A better understanding of the pathogenesis of atopic dermatitis in recent years has contributed to the development of new therapeutic approaches that target specific pathophysiological pathways. Skin barrier dysfunction and immunological abnormalities are critical in the pathogenesis of AD. Recently, the importance of the downregulation of epidermal differentiation complex (EDC) molecules caused by external and internal stimuli has been extensively emphasized. The purpose of this review is to discuss the innovations in the therapy of atopic dermatitis, including biologics, small molecule therapies, and other drugs by highlighting regulatory mechanisms of skin barrier-related molecules, such as filaggrin (FLG) as a crucial pathway implicated in AD pathogenesis. Keywords: atopic dermatitis; skin barrier; filaggrin; biologicals; small molecule therapies Citation: D˛ebi´nska,A. New Treatments for Atopic Dermatitis 1. Introduction Targeting Skin Barrier Repair via the Atopic dermatitis (AD) is one of the most common chronic inflammatory skin disor- Regulation of FLG Expression. -

Biological Therapy in Systemic Sclerosis
Chapter 7 Biological Therapy in Systemic Sclerosis Joana Caetano, Susana Oliveira and JoanaJosé Delgado Caetano, Alves Susana Oliveira and AdditionalJosé Delgado information Alves is available at the end of the chapter Additional information is available at the end of the chapter http://dx.doi.org/10.5772/intechopen.69326 Abstract Systemic sclerosis is the autoimmune connective tissue disease with the highest morbid- ity and mortality, through the combination of inflammation, vasculopathy and fibrosis leading to severe internal organ involvement. Currently, there are no approved disease- modifying therapies, and treatment is based on organ-specific treatment and broad immu- nosuppression, with disappointing long-term results in most cases. Recent research has helped to improve knowledge of the pathogenesis of systemic sclerosis and to optimize treatment based on specific physiopathological targets, and a new era of biological agents in systemic sclerosis has now begun. Promising results are emerging from targeting spe- cific cytokine signalling, especially IL-6, and cellular subpopulations such as B cells, with anti-CD20 therapy, and T-cells, with inhibition of T-cell co-stimulation. Other approaches under evaluation are based on the modulation of profibrotic pathways by anti-TGF-β agents. In this chapter, we discuss the available evidence to support the use of each biologi- cal agent in systemic sclerosis based on data from basic and translational research and on results from clinical studies. Keywords: systemic sclerosis, biological therapy, cytokines, immune dysfunction, targeted treatment 1. Introduction Systemic sclerosis (SSc) is a rare multisystem connective tissue disease in which inflamma- tion, fibrosis, vasculopathy and autoimmunity are the principal features leading to diverse organ-based injuries [1]. -

Atopic Dermatitis (AD)
This activity is provided by PRIME Education. There is no fee to participate. This activity is supported by education grants from AbbVie, Inc., Sanofi Genzyme and Regeneron Pharmaceuticals. © 2019 PRIME® Education, LLC. All Rights Reserved.. Overview This downloadable fact‐sheet provides an easy‐to‐follow collection of the latest evidence shaping the treatment and management of psoriasis (PsO) and atopic dermatitis (AD). Learn about validated tools, evidence‐based strategies, and new and emerging targeted therapies that can be incorporated in daily practice to improve outcomes for patients with these conditions. © 2019 PRIME® Education, LLC. All Rights Reserved.. 2 1 Learning Objectives • Identify major barriers to evidence‐based treatment and management in federal and public sectors • Implement appropriate methods for diagnosis and assessment of disease activity • Assess current evidence on targeted biologic and small‐molecule therapies to guide treatment decisions for patients with moderate to severe disease • Monitor treatment responses according to treat‐to‐target principles and methods • Apply current evidence and guidelines to inform treatment decisions for patients with inadequate responses to initial therapies • Incorporate patient‐reported outcomes and shared decision‐making into clinical practice • Apply effective strategies for multidisciplinary care coordination and shared patient management © 2019 PRIME® Education, LLC. All Rights Reserved.. 3 Accreditation In support of improving patient care, PRIME® is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC) to provide continuing education for the healthcare team. This activity was planned by and for the healthcare team, and learners will receive 2.25 Interprofessional Continuing Education (IPCE) credits for learning and change. -

Atopic Dermatitis: an Expanding Therapeutic Pipeline for a Complex Disease
REVIEWS Atopic dermatitis: an expanding therapeutic pipeline for a complex disease Thomas Bieber 1,2,3 Abstract | Atopic dermatitis (AD) is a common chronic inflammatory skin disease with a complex pathophysiology that underlies a wide spectrum of clinical phenotypes. AD remains challenging to treat owing to the limited response to available therapies. However, recent advances in understanding of disease mechanisms have led to the discovery of novel potential therapeutic targets and drug candidates. In addition to regulatory approval for the IL-4Ra inhibitor dupilumab, the anti- IL-13 inhibitor tralokinumab and the JAK1/2 inhibitor baricitinib in Europe, there are now more than 70 new compounds in development. This Review assesses the various strategies and novel agents currently being investigated for AD and highlights the potential for a precision medicine approach to enable prevention and more effective long-term control of this complex disease. Atopic disorders Atopic dermatitis (AD) is the most common chronic inhibitors tacrolimus and pimecrolimus and more 1,2 A group of disorders having in inflammatory skin disease . About 80% of disease cases recently the phosphodiesterase 4 (PDE4) inhibitor cris- common a genetic tendency to typically start in infancy or childhood, with the remain- aborole. For the more severe forms of AD, besides the develop IgE- mediated allergic der developing during adulthood. Whereas the point use of ultraviolet light, current therapeutic guidelines reactions. These are atopic dermatitis, food allergy, allergic prevalence in children varies from 2.7% to 20.1% across suggest ciclosporin A, methotrexate, azathioprine and 3,4 rhino- conjunctivitis and countries, it ranges from 2.1% to 4.9% in adults . -

Treatment of Diffuse Cutaneous Systemic Sclerosis with Hyperimmune Caprine Serum
University College London (UCL) MD (Res) Degree Treatment of Diffuse Cutaneous Systemic Sclerosis with Hyperimmune Caprine Serum DR. NIAMH PATRICIA QUILLINAN 1 Declaration I, Niamh Patricia Quillinan, confirm that the work presented in this thesis is my own. Where information has been derived from other sources, I confirm that this has been indicated in the thesis. Signed: ___________________________________ Date: 17/03/2016 2 Abstract Systemic sclerosis (SSc) is a multisystem autoimmune rheumatic disorder with high morbidity and the highest case specific mortality of the rheumatic diseases. There is no currently approved unequivocally effective treatment for SSc and therefore there is a huge unmet medical need for novel and effective therapies. Hyperimmune caprine serum (HCS) is a goat serum extract derivative produced from goats vaccinated with a detergent-inactivated HIV viral lysate. It contains caprine immunoglobulins and small molecular weight proteins as well as a CRH, α-2 macroglobulin (α-2M) and lipoprotein-related peptide-1 complex. In this thesis we explore the hypothesis that hyperimmune caprine serum improves skin and other measures of disease severity in established dcSSc by modulating immunological function that determines persistence of clinical disease. This hypothesis is explored through 1) a prospective clinical trial, 2) long-term clinical use and 3) detailed assessment of serum growth factors and cytokines, as well as established and exploratory markers of disease. The primary objective of the clinical trial was to explore safety and tolerability of HCS in established diffuse cutaneous systemic sclerosis (dcSSc). Secondary objectives included assessment of potential efficacy and biological activity and exploration of candidate biomarkers. There were no safety concerns and frequency of adverse events was not different between HCS and placebo group. -

Clinical Research Newsletter for Colleagues in the Community
Clinical Research Newsletter for Colleagues in the Community Welcome to the Winter 2017 issue of the Stanford Cancer surgery, robotic surgery, stereotactic radiosurgery such as Institute Clinical Research Newsletter. This quarterly CyberKnife®, microvascular reconstruction, intraoperative publication is designed to inform our colleagues in the radiation therapy (IORT), along with new chemotherapy trials. medical community, and especially physicians who are The Stanford Cancer Institute Neuro-Oncology Program considering treatment options for their patients with offers Phase I through III trials as well as multidisciplinary, cancer, about current clinical trials available at the Stanford collaborative evaluation and treatment of patients with tumors Cancer Institute, a National Cancer Institute designated of the nervous system. This includes, but is not restricted Comprehensive Cancer Center. Many of these trials provide to; brain metastases, leptomeningeal cancer, glioblastomas access to novel therapies including new “targeted” agents, and less aggressive gliomas, benign brain and spinal tumors, often not available in the community. and base of brain neoplasms including pituitary disorders. As leaders of the Stanford Head and Neck Cancer Care Clinical trials have focused on vaccine therapy, antibody Program, we are delighted to introduce this edition of the therapy, novel chemotherapy agents, radiation sensitizers, newsletter, as it focuses on our Neuro-Oncology, Thoracic novel radiation therapy, and radiosurgery techniques. Oncology, and Developmental Therapeutics programs. The Developmental Therapeutics Program conducts Each of these programs offers cutting-edge clinical trials pharmacokinetic and pharmacodynamic driven first-in- for patients with tumors that can be challenging to treat human trials tailored to make early, informed decisions with current routine care. Weekly multidisciplinary tumor regarding the suitability of novel molecular agents for boards are available for each program. -

(12) Patent Application Publication (10) Pub. No.: US 2017/0172932 A1 Peyman (43) Pub
US 20170172932A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0172932 A1 Peyman (43) Pub. Date: Jun. 22, 2017 (54) EARLY CANCER DETECTION AND A 6LX 39/395 (2006.01) ENHANCED IMMUNOTHERAPY A61R 4I/00 (2006.01) (52) U.S. Cl. (71) Applicant: Gholam A. Peyman, Sun City, AZ CPC .......... A61K 9/50 (2013.01); A61K 39/39558 (US) (2013.01); A61K 4I/0052 (2013.01); A61 K 48/00 (2013.01); A61K 35/17 (2013.01); A61 K (72) Inventor: sham A. Peyman, Sun City, AZ 35/15 (2013.01); A61K 2035/124 (2013.01) (21) Appl. No.: 15/143,981 (57) ABSTRACT (22) Filed: May 2, 2016 A method of therapy for a tumor or other pathology by administering a combination of thermotherapy and immu Related U.S. Application Data notherapy optionally combined with gene delivery. The combination therapy beneficially treats the tumor and pre (63) Continuation-in-part of application No. 14/976,321, vents tumor recurrence, either locally or at a different site, by filed on Dec. 21, 2015. boosting the patient’s immune response both at the time or original therapy and/or for later therapy. With respect to Publication Classification gene delivery, the inventive method may be used in cancer (51) Int. Cl. therapy, but is not limited to such use; it will be appreciated A 6LX 9/50 (2006.01) that the inventive method may be used for gene delivery in A6 IK 35/5 (2006.01) general. The controlled and precise application of thermal A6 IK 4.8/00 (2006.01) energy enhances gene transfer to any cell, whether the cell A 6LX 35/7 (2006.01) is a neoplastic cell, a pre-neoplastic cell, or a normal cell. -

What's New in Eczema Management
What’s New in Eczema Management: Practice Pearls for the Family Physician This educational activity is jointly provided by the North Carolina Academy of Family Physicians (NCAFP) and Spire Learning. This activity is supported by an educational grant from Pfizer Inc. What’s New in Eczema Management: Practice Pearls for the Family Physician PROGRAM OVERVIEW This live meeting series will address the latest in the care and management of pediatric and adult dermatitis as well as strategies to individualize treatment. TARGET AUDIENCE Family physicians LEARNING OBJECTIVES At the conclusion of this live activity, family physicians should be better able to: • Recognize the clinical features and characteristic age distribution patterns of atopic dermatitis (AD) • Describe the role of skin barrier dysfunction, immune dysregulation, and environmental factors in the pathogenesis of AD • Individualize AD management regimens according to age, location, disease severity, response to treatment, and quality-of-life (QOL) concerns • Educate patients and families about the safe and appropriate use of skin-directed therapies for the treatment of AD ACCREDITATION AND DISCLAIMER STATEMENTS This live activity, What’s New in Eczema Management: Practice Pearls for the Family Physician has been reviewed and is acceptable for up to 1.00 Prescribed credit(s) by the American Academy of Family Physicians. Physicians should claim only the credit commensurate with the extent of their participation in the activity. AMA/AAFP Equivalency: AAFP Prescribed credit is accepted by the American Medical Association as equivalent to AMA PRA Category 1 Credit(s)™ toward the AMA Physician’s Recognition Award. When applying for the AMA PRA, Prescribed credit earned must be reported as Prescribed credit, not as Category 1. -
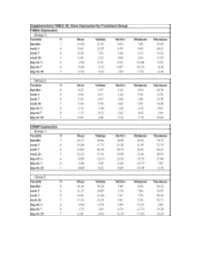
Fresolimumab Suppl Methods
Supplementary Figure 1. Fresolimumab treated patients show decreased biomarkers and skin scores. Graphs show thrombopondin-1 (THBS1, panels a and b) and cartilage oligomeric protein (COMP, panels c and d) gene expression, and MRSS (panels e and f) from study patients in group 1, receiving two doses of 1 mg/kg fresolimumab, (a, c, and e) and group 2, receiving one dose of 5 mg/kg fresolimumab (b, d and f). Line graphs show changes in individual patients over time. A B C Supplementary Figure 2: Biomarker gene expression correlates with the local skin score. The local forearm skin score correlates highly with the MRSS (panel a), as well as THBS1 (panel b) and COMP (panel c) gene expression. Supplementary Figure 3. Autoantibody levels measured at baseline and 11 weeks after fresolimumab treatment. Anti-RNA polymerase III levels for anti-RNA polymerase III- positive patients are shown in blue. Anti-Scl70 levels for anti-Scl70-positive patients are shown in red. Autoantibody levels in two of the four anti-Scl70-positive patients lie on top of the other two. Supplementary Figure 4. Disease duration compared to the change in MRSS 7 weeks after fresolimumab treatment. The change in MRSS after fresolimumab treatment showed no correlation with the disease duration (r2 = 0.00063) Supplementary Figure 5A. Immunohistochemical Supplementary Figure 5B. Skin collagen staining of plasminogen activator protein-1 (PAI- thickness. Based on trichrome staining, dermal 1/serpine1). Each patient is represented by a thickness was measured from the dermal-epidermal different color/style of marker. The median of the junction to subcutaneous fat. -

Epithelial–Mesenchymal Transition in Crohn's Disease
REVIEW Epithelial–mesenchymal transition in Crohn’s disease H Jiang1, J Shen1 and Z Ran1 Crohn’s disease (CD) is often accompanied by the complications of intestinal strictures and fistulas. These complications remain obstacles in CD treatment. In recent years, the importance of epithelial–mesenchymal transition in the pathogenesis of CD-associated fistulas and intestinal fibrosis has become apparent. Epithelial–mesenchymal transition refers to a dynamic change, wherein epithelial cells lose their polarity and adherence and acquire migratory function and fibroblast features. During formation of CD-associated fistulas, intestinal epithelial cells dislocate from the basement membrane and migrate to the lining of the fistula tracts, where they convert into transitional cells as a compensatory response under the insufficient wound healing condition. In CD-associated intestinal fibrosis, epithelial–mesenchymal transition may serve as a source of new fibroblasts and consequently lead to overproduction of extracellular matrix. In this review, we present current knowledge of epithelial–mesenchymal transition and its role in the pathogenesis of CD in order to highlight new therapy targets for the associated complications. INTRODUCTION and fistula formation in CD.6,7 EMT was first identified in the Therapeutic strategies for Crohn’s disease (CD) have progressed 1960s from the pioneering work of Elizabeth Hay, who remarkably in the past decade.1 From 1991 to 2014, the rate of described transformation of epithelial cells to mesenchymal hospitalization and surgical intervention for CD decreased; cells in the chick primitive streak during embryogenesis.8 however, despite the use of advanced drugs such as immuno- Generally, in EMT, epithelial cells demonstrate plasticity and modulators and biologics, the progression to complicated disease lose epithelial markers like E-cadherin and catenins.