[Thesis Title Page]
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The 2014 Golden Gate National Parks Bioblitz - Data Management and the Event Species List Achieving a Quality Dataset from a Large Scale Event
National Park Service U.S. Department of the Interior Natural Resource Stewardship and Science The 2014 Golden Gate National Parks BioBlitz - Data Management and the Event Species List Achieving a Quality Dataset from a Large Scale Event Natural Resource Report NPS/GOGA/NRR—2016/1147 ON THIS PAGE Photograph of BioBlitz participants conducting data entry into iNaturalist. Photograph courtesy of the National Park Service. ON THE COVER Photograph of BioBlitz participants collecting aquatic species data in the Presidio of San Francisco. Photograph courtesy of National Park Service. The 2014 Golden Gate National Parks BioBlitz - Data Management and the Event Species List Achieving a Quality Dataset from a Large Scale Event Natural Resource Report NPS/GOGA/NRR—2016/1147 Elizabeth Edson1, Michelle O’Herron1, Alison Forrestel2, Daniel George3 1Golden Gate Parks Conservancy Building 201 Fort Mason San Francisco, CA 94129 2National Park Service. Golden Gate National Recreation Area Fort Cronkhite, Bldg. 1061 Sausalito, CA 94965 3National Park Service. San Francisco Bay Area Network Inventory & Monitoring Program Manager Fort Cronkhite, Bldg. 1063 Sausalito, CA 94965 March 2016 U.S. Department of the Interior National Park Service Natural Resource Stewardship and Science Fort Collins, Colorado The National Park Service, Natural Resource Stewardship and Science office in Fort Collins, Colorado, publishes a range of reports that address natural resource topics. These reports are of interest and applicability to a broad audience in the National Park Service and others in natural resource management, including scientists, conservation and environmental constituencies, and the public. The Natural Resource Report Series is used to disseminate comprehensive information and analysis about natural resources and related topics concerning lands managed by the National Park Service. -

California Saltwater Sport Fishing Regulations
2017–2018 CALIFORNIA SALTWATER SPORT FISHING REGULATIONS For Ocean Sport Fishing in California Effective March 1, 2017 through February 28, 2018 13 2017–2018 CALIFORNIA SALTWATER SPORT FISHING REGULATIONS Groundfish Regulation Tables Contents What’s New for 2017? ............................................................. 4 24 License Information ................................................................ 5 Sport Fishing License Fees ..................................................... 8 Keeping Up With In-Season Groundfish Regulation Changes .... 11 Map of Groundfish Management Areas ...................................12 Summaries of Recreational Groundfish Regulations ..................13 General Provisions and Definitions ......................................... 20 General Ocean Fishing Regulations ��������������������������������������� 24 Fin Fish — General ................................................................ 24 General Ocean Fishing Fin Fish — Minimum Size Limits, Bag and Possession Limits, and Seasons ......................................................... 24 Fin Fish—Gear Restrictions ................................................... 33 Invertebrates ........................................................................ 34 34 Mollusks ............................................................................34 Crustaceans .......................................................................36 Non-commercial Use of Marine Plants .................................... 38 Marine Protected Areas and Other -

CHECKLIST and BIOGEOGRAPHY of FISHES from GUADALUPE ISLAND, WESTERN MEXICO Héctor Reyes-Bonilla, Arturo Ayala-Bocos, Luis E
ReyeS-BONIllA eT Al: CheCklIST AND BIOgeOgRAphy Of fISheS fROm gUADAlUpe ISlAND CalCOfI Rep., Vol. 51, 2010 CHECKLIST AND BIOGEOGRAPHY OF FISHES FROM GUADALUPE ISLAND, WESTERN MEXICO Héctor REyES-BONILLA, Arturo AyALA-BOCOS, LUIS E. Calderon-AGUILERA SAúL GONzáLEz-Romero, ISRAEL SáNCHEz-ALCántara Centro de Investigación Científica y de Educación Superior de Ensenada AND MARIANA Walther MENDOzA Carretera Tijuana - Ensenada # 3918, zona Playitas, C.P. 22860 Universidad Autónoma de Baja California Sur Ensenada, B.C., México Departamento de Biología Marina Tel: +52 646 1750500, ext. 25257; Fax: +52 646 Apartado postal 19-B, CP 23080 [email protected] La Paz, B.C.S., México. Tel: (612) 123-8800, ext. 4160; Fax: (612) 123-8819 NADIA C. Olivares-BAñUELOS [email protected] Reserva de la Biosfera Isla Guadalupe Comisión Nacional de áreas Naturales Protegidas yULIANA R. BEDOLLA-GUzMáN AND Avenida del Puerto 375, local 30 Arturo RAMíREz-VALDEz Fraccionamiento Playas de Ensenada, C.P. 22880 Universidad Autónoma de Baja California Ensenada, B.C., México Facultad de Ciencias Marinas, Instituto de Investigaciones Oceanológicas Universidad Autónoma de Baja California, Carr. Tijuana-Ensenada km. 107, Apartado postal 453, C.P. 22890 Ensenada, B.C., México ABSTRACT recognized the biological and ecological significance of Guadalupe Island, off Baja California, México, is Guadalupe Island, and declared it a Biosphere Reserve an important fishing area which also harbors high (SEMARNAT 2005). marine biodiversity. Based on field data, literature Guadalupe Island is isolated, far away from the main- reviews, and scientific collection records, we pres- land and has limited logistic facilities to conduct scien- ent a comprehensive checklist of the local fish fauna, tific studies. -

Fish Bulletin No. 109. the Barred Surfperch (Amphistichus Argenteus Agassiz) in Southern California
UC San Diego Fish Bulletin Title Fish Bulletin No. 109. The Barred Surfperch (Amphistichus argenteus Agassiz) in Southern California Permalink https://escholarship.org/uc/item/9fh0623k Authors Carlisle, John G, Jr. Schott, Jack W Abramson, Norman J Publication Date 1960 eScholarship.org Powered by the California Digital Library University of California STATE OF CALIFORNIA DEPARTMENT OF FISH AND GAME MARINE RESOURCES OPERATIONS FISH BULLETIN No. 109 The Barred Surfperch (Amphistichus argenteus Agassiz) in Southern Califor- nia By JOHN G. CARLISLE, JR., JACK W. SCHOTT and NORMAN J. ABRAMSON 1960 1 2 3 4 ACKNOWLEDGMENTS The Surf Fishing Investigation received a great deal of help in the conduct of its field work. The arduous task of beach seining all year around was shared by many members of the California State Fisheries Laboratory staff; we are particularly grateful to Mr. Parke H. Young and Mr. John L. Baxter for their willing and continued help throughout the years. Mr. Frederick B. Hagerman was project leader for the first year of the investigation, until his recall into the Air Force, and he gave the project an excellent start. Many others gave help and advice, notably Mr. John E. Fitch, Mr. Phil M. Roedel, Mr. David C. Joseph, and Dr. F. N. Clark of this laboratory. Dr. Carl L. Hubbs of Scripps Institution of Oceanography at La Jolla gave valuable advice, and we are indebted to the late Mr. Conrad Limbaugh of the same institution for accounts of his observations on surf fishes, and for SCUBA diving instructions. The project was fortunate in securing able seasonal help, particularly from Mr. -
Appendix E: Fish Species List
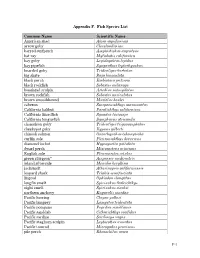
Appendix F. Fish Species List Common Name Scientific Name American shad Alosa sapidissima arrow goby Clevelandia ios barred surfperch Amphistichus argenteus bat ray Myliobatis californica bay goby Lepidogobius lepidus bay pipefish Syngnathus leptorhynchus bearded goby Tridentiger barbatus big skate Raja binoculata black perch Embiotoca jacksoni black rockfish Sebastes melanops bonehead sculpin Artedius notospilotus brown rockfish Sebastes auriculatus brown smoothhound Mustelus henlei cabezon Scorpaenichthys marmoratus California halibut Paralichthys californicus California lizardfish Synodus lucioceps California tonguefish Symphurus atricauda chameleon goby Tridentiger trigonocephalus cheekspot goby Ilypnus gilberti chinook salmon Oncorhynchus tshawytscha curlfin sole Pleuronichthys decurrens diamond turbot Hypsopsetta guttulata dwarf perch Micrometrus minimus English sole Pleuronectes vetulus green sturgeon* Acipenser medirostris inland silverside Menidia beryllina jacksmelt Atherinopsis californiensis leopard shark Triakis semifasciata lingcod Ophiodon elongatus longfin smelt Spirinchus thaleichthys night smelt Spirinchus starksi northern anchovy Engraulis mordax Pacific herring Clupea pallasi Pacific lamprey Lampetra tridentata Pacific pompano Peprilus simillimus Pacific sanddab Citharichthys sordidus Pacific sardine Sardinops sagax Pacific staghorn sculpin Leptocottus armatus Pacific tomcod Microgadus proximus pile perch Rhacochilus vacca F-1 plainfin midshipman Porichthys notatus rainwater killifish Lucania parva river lamprey Lampetra -

Environmental DNA Reveals the Fine-Grained and Hierarchical
www.nature.com/scientificreports OPEN Environmental DNA reveals the fne‑grained and hierarchical spatial structure of kelp forest fsh communities Thomas Lamy 1,2*, Kathleen J. Pitz 3, Francisco P. Chavez3, Christie E. Yorke1 & Robert J. Miller1 Biodiversity is changing at an accelerating rate at both local and regional scales. Beta diversity, which quantifes species turnover between these two scales, is emerging as a key driver of ecosystem function that can inform spatial conservation. Yet measuring biodiversity remains a major challenge, especially in aquatic ecosystems. Decoding environmental DNA (eDNA) left behind by organisms ofers the possibility of detecting species sans direct observation, a Rosetta Stone for biodiversity. While eDNA has proven useful to illuminate diversity in aquatic ecosystems, its utility for measuring beta diversity over spatial scales small enough to be relevant to conservation purposes is poorly known. Here we tested how eDNA performs relative to underwater visual census (UVC) to evaluate beta diversity of marine communities. We paired UVC with 12S eDNA metabarcoding and used a spatially structured hierarchical sampling design to assess key spatial metrics of fsh communities on temperate rocky reefs in southern California. eDNA provided a more‑detailed picture of the main sources of spatial variation in both taxonomic richness and community turnover, which primarily arose due to strong species fltering within and among rocky reefs. As expected, eDNA detected more taxa at the regional scale (69 vs. 38) which accumulated quickly with space and plateaued at only ~ 11 samples. Conversely, the discovery rate of new taxa was slower with no sign of saturation for UVC. -

Status of the Pacific Herring, Clupea Harengus Pallasii, Resource in California 1972 to 1980
STATE OF CALIFORNIA THE RESOURCES AGENCY DEPARTMENT OF FISH AND GAME FISH BULLETIN 171 Status of The Pacific Herring, Clupea Harengus Pallasii, Resource In Califor- nia 1972 to 1980 by Jerome D. Spratt 1981 1 ABSTRACT The California Department of Fish and Game has conducted periodic studies on Pacific herring since 1953. This re- port concentrates on the period from 1972 through 1980 during which the herring fishery underwent a dramatic re- surgence due to the opening of a lucrative market for herring roe in Japan. The spawning biomass of Pacific herring was estimated by determining numbers of eggs spawned and using pre- viously derived estimates of eggs per gram of fish to convert this figure to short tons of herring. Spawning biomass estimates for Tomales Bay ranged from 4,728 tons in the 1974–75 season to 22,163 tons in the 1977–78 season. Es- timates for San Francisco Bay ranged from 6,179 tons in 1973–74 season to 52,869 tons in the 1979–80 season. Sampling the roe fishery catch in Tomales and San Francisco Bays revealed that age 2 and 3 herring dominated the round haul fishery, and ages 5 and 6 dominated the gill net fishery. Gill nets consistently caught larger herring and a higher percentage of females than round haul nets. Comparison of length at age of herring from Tomales and San Francisco Bays revealed a statistical difference in growth rates between populations of the two bays. Tomales Bay herring are larger at a given age than San Francisco Bay herring. Spawning time was related to the tidal cycle in San Francisco Bay. -

Terrestrial and Marine Biological Resource Information
APPENDIX C Terrestrial and Marine Biological Resource Information Appendix C1 Resource Agency Coordination Appendix C2 Marine Biological Resources Report APPENDIX C1 RESOURCE AGENCY COORDINATION 1 The ICF terrestrial biological team coordinated with relevant resource agencies to discuss 2 sensitive biological resources expected within the terrestrial biological study area (BSA). 3 A summary of agency communications and site visits is provided below. 4 California Department of Fish and Wildlife: On July 30, 2020, ICF held a conference 5 call with Greg O’Connell (Environmental Scientist) and Corianna Flannery (Environmental 6 Scientist) to discuss Project design and potential biological concerns regarding the 7 Eureka Subsea Fiber Optic Cables Project (Project). Mr. O’Connell discussed the 8 importance of considering the western bumble bee. Ms. Flannery discussed the 9 importance of the hard ocean floor substrate and asked how the cable would be secured 10 to the ocean floor to reduce or eliminate scour. The western bumble bee has been 11 evaluated in the Biological Resources section of the main document, and direct and 12 indirect impacts are avoided. The Project Description describes in detail how the cable 13 would be installed on the ocean floor, the importance of the hard bottom substrate, and 14 the need for avoidance. 15 Consultation Outcomes: 16 • The Project was designed to avoid hard bottom substrate, and RTI Infrastructure 17 (RTI) conducted surveys of the ocean floor to ensure that proper routing of the 18 cable would occur. 19 • Ms. Flannery will be copied on all communications with the National Marine 20 Fisheries Service 21 California Department of Fish and Wildlife: On August 7, 2020, ICF held a conference 22 call with Greg O’Connell to discuss a site assessment and survey approach for the 23 western bumble bee. -

UC San Diego UC San Diego Electronic Theses and Dissertations
UC San Diego UC San Diego Electronic Theses and Dissertations Title A historical perspective of California recreational fisheries using a new database of "trophy" fish records (1966-2013), combined with fisheries analyses of three species in the genus Paralabrax Permalink https://escholarship.org/uc/item/1g40s1h0 Author Bellquist, Lyall F. Publication Date 2015 Peer reviewed|Thesis/dissertation eScholarship.org Powered by the California Digital Library University of California UNIVERSITY OF CALIFORNIA, SAN DIEGO A historical perspective of California recreational fisheries using a new database of “trophy” fish records (1966-2013), combined with fisheries analyses of three species in the genus Paralabrax A dissertation submitted in partial satisfaction of the requirements for the degree Doctor of Philosophy in Marine Biology by Lyall F. Bellquist Committee in charge: Brice Semmens, Chair Richard Carson David Checkley Philip Hastings Ed Parnell 2015 Copyright Lyall F. Bellquist, 2015 All rights reserved. The Dissertation of Lyall F. Bellquist is approved, and it is acceptable in quality and form for publication on microfilm and electronically: ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ Chair University of California, San -

UC Santa Barbara UC Santa Barbara Electronic Theses and Dissertations
UC Santa Barbara UC Santa Barbara Electronic Theses and Dissertations Title The role of fluctuating food supply on recruitment, survival and population dynamics in the sea Permalink https://escholarship.org/uc/item/6x20t65m Author Okamoto, Daniel K. Publication Date 2014 Peer reviewed|Thesis/dissertation eScholarship.org Powered by the California Digital Library University of California UNIVERSITY OF CALIFORNIA Santa Barbara The role of fluctuating food supply on recruitment, survival and population dynamics in the sea A Dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Ecology, Evolution and Marine Biology by Daniel Kenji Okamoto Committee in Charge: Professor Sally J. Holbrook, Chair Dr Daniel C. Reed Professor Russell J. Schmitt Professor Cherie J. Briggs December 2014 The Dissertation of Daniel Kenji Okamoto is approved: Dr Daniel C. Reed Professor Russell J. Schmitt Professor Cherie J. Briggs Professor Sally J. Holbrook, Committee Chair September 2014 for Christian & A.E. Taylor iii Acknowledgements Throughout my tenure at this University, I've had the blessing of a committee in- vested in and dedicated to my success. Watching friends and colleagues float in the wake of advisors' absentia always made me appreciate my own mentors. Dan Reed, Sally Hol- brook, and Russ Schmitt let me follow my own curiosity and pursuits, and provided direction, time, criticism (and data) to keep me focused and productive. Cherie Briggs too provided critical and and remarkable discussions that led to moments of redirection in my research. Many groups financially supported my research and graduate career. Foremost among these was the National Science Foundation in support of the Santa Barbara Coastal Long Term Ecological Research (LTER) site. -

Humboldt Bay Fishes
Humboldt Bay Fishes ><((((º>`·._ .·´¯`·. _ .·´¯`·. ><((((º> ·´¯`·._.·´¯`·.. ><((((º>`·._ .·´¯`·. _ .·´¯`·. ><((((º> Acknowledgements The Humboldt Bay Harbor District would like to offer our sincere thanks and appreciation to the authors and photographers who have allowed us to use their work in this report. Photography and Illustrations We would like to thank the photographers and illustrators who have so graciously donated the use of their images for this publication. Andrey Dolgor Dan Gotshall Polar Research Institute of Marine Sea Challengers, Inc. Fisheries And Oceanography [email protected] [email protected] Michael Lanboeuf Milton Love [email protected] Marine Science Institute [email protected] Stephen Metherell Jacques Moreau [email protected] [email protected] Bernd Ueberschaer Clinton Bauder [email protected] [email protected] Fish descriptions contained in this report are from: Froese, R. and Pauly, D. Editors. 2003 FishBase. Worldwide Web electronic publication. http://www.fishbase.org/ 13 August 2003 Photographer Fish Photographer Bauder, Clinton wolf-eel Gotshall, Daniel W scalyhead sculpin Bauder, Clinton blackeye goby Gotshall, Daniel W speckled sanddab Bauder, Clinton spotted cusk-eel Gotshall, Daniel W. bocaccio Bauder, Clinton tube-snout Gotshall, Daniel W. brown rockfish Gotshall, Daniel W. yellowtail rockfish Flescher, Don american shad Gotshall, Daniel W. dover sole Flescher, Don stripped bass Gotshall, Daniel W. pacific sanddab Gotshall, Daniel W. kelp greenling Garcia-Franco, Mauricio louvar -

Open Ocean Intake Effects Study
City of Santa Cruz Water Department & Soquel Creek Water District scwd2 Desalination Program Open Ocean Intake Effects Study December 2010 Submitted to: Ms. Heidi Luckenbach City of Santa Cruz 212 Locust Street Santa Cruz, CA 95060 Prepared by: Environmental ESLO2010-017.1 [Blank Page] ACKNOWLEDGEMENTS Tenera Environmental wishes to acknowledge the valuable contributions of the Santa Cruz Water Department, Soquel Creek Water District, and scwd² Task Force in conducting the Open Ocean Intake Effects Study. Specifically, Tenera would like to acknowledge the efforts of: City of Santa Cruz Water Department Soquel Creek Water District Bill Kocher, Director Laura Brown, General Manager Linette Almond, Engineering Manager Melanie Mow Schumacher, Public Information Heidi R. Luckenbach, Program Coordinator Coordinator Leah Van Der Maaten, Associate Engineer Catherine Borrowman, Professional and Technical scwd² Task Force Assistant Ryan Coonerty Todd Reynolds, Kennedy/Jenks and scwd² Bruce Daniels Technical Advisor Bruce Jaffe Dan Kriege Thomas LaHue Don Lane Cynthia Mathews Mike Rotkin Ed Porter Tenera’s project team included the following members: David L. Mayer, Ph.D., Tenera Environmental President and Principal Scientist John Steinbeck, Tenera Environmental Vice President and Principal Scientist Carol Raifsnider, Tenera Environmental Director of Operations and Principal Scientist Technical review and advice was provided by: Pete Raimondi, Ph.D., UCSC, Professor of Ecology and Evolutionary Biology in the Earth and Marine Sciences Dept. Gregor