Human ROS1 Fusion Gene Detection PCR Kit
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

RET Gene Fusions in Malignancies of the Thyroid and Other Tissues
G C A T T A C G G C A T genes Review RET Gene Fusions in Malignancies of the Thyroid and Other Tissues Massimo Santoro 1,*, Marialuisa Moccia 1, Giorgia Federico 1 and Francesca Carlomagno 1,2 1 Department of Molecular Medicine and Medical Biotechnology, University of Naples “Federico II”, 80131 Naples, Italy; [email protected] (M.M.); [email protected] (G.F.); [email protected] (F.C.) 2 Institute of Endocrinology and Experimental Oncology of the CNR, 80131 Naples, Italy * Correspondence: [email protected] Received: 10 March 2020; Accepted: 12 April 2020; Published: 15 April 2020 Abstract: Following the identification of the BCR-ABL1 (Breakpoint Cluster Region-ABelson murine Leukemia) fusion in chronic myelogenous leukemia, gene fusions generating chimeric oncoproteins have been recognized as common genomic structural variations in human malignancies. This is, in particular, a frequent mechanism in the oncogenic conversion of protein kinases. Gene fusion was the first mechanism identified for the oncogenic activation of the receptor tyrosine kinase RET (REarranged during Transfection), initially discovered in papillary thyroid carcinoma (PTC). More recently, the advent of highly sensitive massive parallel (next generation sequencing, NGS) sequencing of tumor DNA or cell-free (cfDNA) circulating tumor DNA, allowed for the detection of RET fusions in many other solid and hematopoietic malignancies. This review summarizes the role of RET fusions in the pathogenesis of human cancer. Keywords: kinase; tyrosine kinase inhibitor; targeted therapy; thyroid cancer 1. The RET Receptor RET (REarranged during Transfection) was initially isolated as a rearranged oncoprotein upon the transfection of a human lymphoma DNA [1]. -
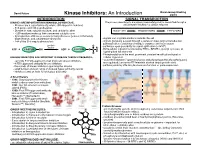
Kinase Inhibitors: an Introduction
David Peters Baran Group Meeting Kinase Inhibitors: An Introduction 2/2/19 INTRODUCTION SIGNAL TRANSDUCTION KINASES ARE IMPORTANT IN HUMAN BIOLOGY/DISEASE: The process describing how a signal (chemical/physical) is transmitted through a - Kinases are a superfamily of proteins (5th largest in humans) cell ultimately resulting in a cellular response - 518 genes and 106 pseudogenes - Diverse in size, subunit structure, and cellular location RECEPTOR TRANSDUCERS EFFECTORS - ~260 residues make up their conserved catalytic core - dysregulation of kinases occurs in many diseases (cancer, inflamatory, degenerative, and autoimmune diseases) - signals can originate inside or outside the cell - 244 of the 518 map to disease loci - signals generally passed through a series of steps (signal transduction pathway) often consisting of multiple enzymes and messengers protein O - pathways open possibility for signal aplification (>1x106) kinase ATP + PROTEIN OH ADP + PROTEIN O P O - Extracellular signals transduced by RTKs, GPCR’s, guanlyl cyclases, or ligand-gated Ion channels O - Phosphorylation is the most prominent covalent modification/signal in KINASES INHIBITORS ARE IMPORTANT IN DISEASE THERAPY/RESEARCH: cellular regulation - currently 51 FDA approved small molecule kinase inhibitors - “converter enzymes” (protein kinases and phosphoprotein phosphorlyases) - 4 FDA approved antibody kinase inhibitors are regulated; conserve ATP/maintain desired target protein state - thousands of known inhibitors spanning the kinome - pathway ends by affecting biomolecule -

Clinical Activity of Ceritinib in ROS1-Rearranged Non-Small Cell Lung Cancer: Bench to Bedside Report LETTER Vivek Subbiaha,1, David S
LETTER Clinical activity of ceritinib in ROS1-rearranged non-small cell lung cancer: Bench to bedside report LETTER Vivek Subbiaha,1, David S. Honga, and Funda Meric-Bernstama We read with great interest the article by Davare et al. (1) profiling revealed a CD72-ROS1 rearrangement. The on structural insight of ROS1 tyrosine kinase inhibitors. patient was started on crizotinib at 250 mg orally twice Non-small cell lung cancer (NSCLC) is no longer a single daily and exhibited resolution of metastatic disease. disease but a collection of genetically heterogeneous The patient remained disease-free for 13 mo when tumors with different therapeutic options [e.g., aberra- CT-scan showed a relapse with two nodules in the tions in EGFR, BRAF, HER2/Neu, RET, anaplastic lym- right lower lobe. He underwent stereotactic radiation phoma kinase (ALK), and ROS1-rearranged tumors]. therapy. Imaging showed a treatment response but Each of these options has different clinical characteristics new pleural nodules. MRI brain scan showed new in- and therapeutic options with agents that have varying tracranial metastases. After undergoing gamma knife degrees of systemic activity. ROS1 gene rearrange- radiosurgery, the patient was enrolled in an ipilimumab ments define a distinct molecular subgroup of NSCLC. and radiation trial (NCT02239900). His disease pro- ROS1 rearrangement leads to constitutive ROS1 activa- gressed on ipilimumab. The patient was next enrolled tion and activity of crizotinib against ROS1-rearranged on the “Signature Trial,” a modular phase II study to link NSCLC was noted and crizotinib received regulatory targeted therapy to patients with pathway activated tu- approval for the treatment of ROS1 rearranged NSCLC mors; in this study patients whose tumors have aberra- (2). -

Lung Adenocarcinoma Therapeutic Implications
Patient Name Report Date Tumor Type Smith, James 10 January 2016 Lung adenocarcinoma Date of Birth 01 January 1950 Medical Facility Cancer Center Specimen Received 02 January 2016 Sex Male Ordering Physician Williams, Jane Specimen Site Liver FMI Case # TRF000000 Additional Recipient Not Given Date of Collection 03 January 2016 Medical Record # 100001 Medical Facility ID # 000001 Specimen Type Slide Specimen ID SID-00001 Pathologist Not Provided ABOUT THE TEST: FoundationOne™ is a next-generation sequencing (NGS) based assay that identifies genomic alterations within hundreds of cancer-related genes. PATIENT RESULTS TUMOR TYPE: LUNG ADENOCARCINOMA † 6 genomic alterations Genomic Alterations Identified ROS1 CD74-ROS1 fusion 3 therapies associated with potential clinical benefit CDK4 amplification MDM2 amplification 0 therapies associated with lack of response RICTOR amplification APC S688* 18 clinical trials FGF10 amplification Additional Disease-relevant Genes with No Reportable Alterations Identified† EGFR KRAS ALK BRAF MET RET ERBB2 † For a complete list of the genes assayed and performance specifications, please refer to the Appendix THERAPEUTIC IMPLICATIONS Genomic Alterations FDA-Approved Therapies FDA-Approved Therapies Potential Clinical Trials Detected (in patient’s tumor type) (in another tumor type) ROS1 Ceritinib None Yes, see clinical trials CD74-ROS1 fusion Crizotinib section CDK4 None Palbociclib Yes, see clinical trials amplification section MDM2 None None Yes, see clinical trials amplification section RICTOR None None Yes, see clinical trials amplification SAMPLEsection For more comprehensive information please log on to the Interactive Cancer Explorer™ To set up your Interactive Cancer Explorer account, contact your sales representative or call (888) 988-3639. Electronically Signed by Jeffrey S. -

CT Features and Disease Spread Patterns in ROS1-Rearranged Lung
www.nature.com/scientificreports OPEN CT features and disease spread patterns in ROS1‑rearranged lung adenocarcinomas: comparison with those of EGFR‑mutant or ALK‑rearranged lung adenocarcinomas Jung Han Woo1, Tae Jung Kim1*, Tae Sung Kim1 & Joungho Han2 The purpose of this study was to investigate the diferences in CT characteristics and disease spread patterns between ROS1‑rearranged adenocarcinomas and epidermal growth factor receptor (EGFR)‑mutant or anaplastic lymphoma kinase (ALK)‑rearranged adenocarcinomas. Patients with stage IIIb/IV adenocarcinoma with ROS1 rearrangement, EGFR mutations, or ALK rearrangement were retrospectively identifed. Two radiologists evaluated CT features and disease spread patterns. A multivariable logistic regression model was applied to determine the clinical and CT characteristics that can discriminate between ROS1‑rearranged and EGFR‑mutant or ALK‑ rearranged adenocarcinomas. A cohort of 169 patients was identifed (ROS1 = 23, EGFR = 120, and ALK = 26). Compared to EGFR‑mutant adenocarcinomas, ROS1‑rearranged adenocarcinomas were less likely to have air-bronchogram (p = 0.011) and pleural retraction (p = 0.048) and more likely to have pleural efusion (p = 0.025), pericardial metastases (p < 0.001), intrathoracic and extrathoracic nodal metastases (p = 0.047 and 0.023, respectively), and brain metastases (p = 0.017). Following multivariable analysis, age (OR = 1.06; 95% CI: 1.01, 1.12; p = 0.024), pericardial metastases (OR = 10.50; 95% CI: 2.10, 52.60; p = 0.005), and nodal metastases (OR = 8.55; 95% CI: 1.14, 62.52; p = 0.037) were found to be more common in ROS1‑rearranged tumors than in non‑ROS1‑rearranged tumors. ROS1‑rearranged adenocarcinomas appeared as solid tumors and were associated with young age, pericardial metastases and advanced nodal metastases relative to tumors with EGFR mutations or ALK rearrangement. -

Acquired Resistance to Dasatinib in Lung Cancer Cell Lines Conferred by DDR2 Gatekeeper Mutation and NF1 Loss
Published OnlineFirst December 2, 2013; DOI: 10.1158/1535-7163.MCT-13-0817 Molecular Cancer Cancer Biology and Signal Transduction Therapeutics Acquired Resistance to Dasatinib in Lung Cancer Cell Lines Conferred by DDR2 Gatekeeper Mutation and NF1 Loss Ellen M. Beauchamp1, Brittany A. Woods1,7, Austin M. Dulak1, Li Tan3, Chunxiao Xu1, Nathanael S. Gray2, Adam J. Bass1,6, Kwok-kin Wong1,4, Matthew Meyerson1,5,6, and Peter S. Hammerman1,6 Abstract The treatment of non–small cell lung cancer has evolved dramatically over the past decade with the adoption of widespread use of effective targeted therapies in patients with distinct molecular alterations. In lung squamous cell carcinoma (lung SqCC), recent studies have suggested that DDR2 mutations are a biomarker for therapeutic response to dasatinib and clinical trials are underway testing this hypothesis. Although targeted therapeutics are typically quite effective as initial therapy for patients with lung cancer, nearly all patients develop resistance with long-term exposure to targeted drugs. Here, we use DDR2-dependent lung cancer cell lines to model acquired resistance to dasatinib therapy. We perform targeted exome sequencing to identify two distinct mechanisms of acquired resistance: acquisition of the T654I gatekeeper mutation in DDR2 and loss of NF1. We show that NF1 loss activates a bypass pathway, which confers ERK dependency downstream of RAS activation. These results indicate that acquired resistance to dasatinib can occur via both second-site mutations in DDR2 and by activation of bypass pathways. These data may help to anticipate mechanisms of resistance that may be identified in upcoming clinical trials of anti-DDR2 therapy in lung cancer and suggest strategies to overcome resistance. -

Protein Tyrosine Kinases: Their Roles and Their Targeting in Leukemia
cancers Review Protein Tyrosine Kinases: Their Roles and Their Targeting in Leukemia Kalpana K. Bhanumathy 1,*, Amrutha Balagopal 1, Frederick S. Vizeacoumar 2 , Franco J. Vizeacoumar 1,3, Andrew Freywald 2 and Vincenzo Giambra 4,* 1 Division of Oncology, College of Medicine, University of Saskatchewan, Saskatoon, SK S7N 5E5, Canada; [email protected] (A.B.); [email protected] (F.J.V.) 2 Department of Pathology and Laboratory Medicine, College of Medicine, University of Saskatchewan, Saskatoon, SK S7N 5E5, Canada; [email protected] (F.S.V.); [email protected] (A.F.) 3 Cancer Research Department, Saskatchewan Cancer Agency, 107 Wiggins Road, Saskatoon, SK S7N 5E5, Canada 4 Institute for Stem Cell Biology, Regenerative Medicine and Innovative Therapies (ISBReMIT), Fondazione IRCCS Casa Sollievo della Sofferenza, 71013 San Giovanni Rotondo, FG, Italy * Correspondence: [email protected] (K.K.B.); [email protected] (V.G.); Tel.: +1-(306)-716-7456 (K.K.B.); +39-0882-416574 (V.G.) Simple Summary: Protein phosphorylation is a key regulatory mechanism that controls a wide variety of cellular responses. This process is catalysed by the members of the protein kinase su- perfamily that are classified into two main families based on their ability to phosphorylate either tyrosine or serine and threonine residues in their substrates. Massive research efforts have been invested in dissecting the functions of tyrosine kinases, revealing their importance in the initiation and progression of human malignancies. Based on these investigations, numerous tyrosine kinase inhibitors have been included in clinical protocols and proved to be effective in targeted therapies for various haematological malignancies. -

Genetic Testing for Germline Mutations of the RET Proto-Oncogene AHS - M2078
Corporate Medical Policy Genetic Testing for Germline Mutations of the RET Proto-Oncogene AHS - M2078 File Name: genetic_testing_for_germline_mutations_of_the_ret_proto-oncogene Origination: 1/2019 Last CAP Review 3/2021 Next CAP Review: 3/2022 Last Review: 3/2021 Description of Procedure or Service The RET (rearranged during transfection) proto-oncogene encodes a transmembrane receptor tyrosine kinase (Takahashi, Ritz, & Cooper, 1985) that regulates a complex network of signal transduction pathways during development, survival, proliferation, differentiation, and migration of the enteric nervous system progenitor cells (Hedayati, Zarif Yeganeh, Sheikholeslami, & Afsari, 2016). Disruption of RET signaling by mutation, gene rearrangement, overexpression or transcriptional up-regulation of the RET gene is implicated in several human cancers (Plaza-Menacho, Mologni, & McDonald, 2014), most commonly thyroid, but also chronic myelomonocytic leukemia, acute myeloid leukemia, and lung, breast, pancreatic, and colon cancers (Gordon et al., 2018). Mutation of the RET gene in a germline cell results in an autosomal dominant hereditary cancer syndrome, multiple endocrine neoplasia type 2 (MEN2) characterized by medullary thyroid carcinoma (MTC), pheochromocytoma (PHEO), and primary parathyroid hyperplasia (PPTH). (Figlioli, Landi, Romei, Elisei, & Gemignani, 2013). This policy covers genetic testing for germline variants in the RET gene. For information on testing of tumors for RET variants to guide chemotherapy. Related Policies M2109 Molecular Panel Testing of Cancers to Identify Targeted Therapy M2030 Testing for Targeted Therapy of Non-Small-Cell Lung Cancer M2108 Molecular Markers in Fine Needle Aspirates of the Thyroid. ***Note: This Medical Policy is complex and technical. For questions concerning the technical language and/or specific clinical indications for its use, please consult your physician. -
RNA-Based Detection of Gene Fusions in Formalin- Fixed And
Supplementary Materials RNA-Based Detection of Gene Fusions in Formalin- Fixed and Paraffin-Embedded Solid Cancer Samples Martina Kirchner, Olaf Neumann, Anna-Lena Volckmar, Fabian Stögbauer, Michael Allgäuer, Daniel Kazdal, Jan Budczies, Eugen Rempel, Regine Brandt, Suranand Babu Talla, Moritz von Winterfeld, Jonas Leichsenring, Tilmann Bochtler, Alwin Krämer, Christoph Springfeld, Peter Schirmacher, Roland Penzel, Volker Endris and Albrecht Stenzinger Table S1. PCR Primers for gene fusions identified with either the OCAv3- or Archer-panel. Amplicon Fusion Primer Seq Primer Seq [bp] AXL::CAPN15 AXL F CATGGATGAGGGTGGAGGTT CAPN15 R CTGGGCACACGTGAATCAC 178 (A19C2) BRD3::NUTM1 BRD3 F AAGAAACAGGCAGCCAAGTC NUTM1 R CTGGTGGGTCAGAAGTTGGT 217 (B11N2) ESR1-CCDC170 CCDC170 ESR1 F GGAGACTCGCTACTGTGCA CCCAGACTCCTTTCCCAACT 167 (E2C7) R ESR1-QKI (E2Q5) ESR1 F GGAGACTCGCTACTGTGCA QKI R GGCTGGTGATTTAATGTTGGC 197 ETV6::NTRK3 (E5N15) ETV6 F AAGCCCATCAACCTCTCTCA NTRK3 R GGGCTGAGGTTGTAGCACTC 206 FGFR2::INA (F17I2) FGFR2 F CTCCCAGAGACCAACGTTCA INA R GTCCTGGTATTCCCGAAAGGT 148 FNDC3B-PIK3CA PIK3CA FNDC3B F GCAGCTCAGCAGGTTATTCT GTCGTGGAGGCATTGTTCTG 177 (F3P2) R1 GATM::RAF1 (G2R8) GATM F CTTACAACGAATGGGACCCC RAF1 R GTTGGGCTCAGATTGTTGGG 160 GPBP1L1::MAST2 GPBP1L1 CGTAGTGGAGGTGGCACA MAST2 R1 AGGTGATGTGCTAGAGGTCA 178 (G6M4) F1 HNRNPA2B1::ETV1 HNRNPA GGAGGATATGGTGGTGGAGG ETV1 R TTGATTTTCAGTGGCAGGCC 164 (H9E6) 2B1 F TGATGAATCTGGAATTGTTGCT MYB::NFIB (M12N9) MYB12 F NFIB9 R CGTAATTTTGGACATTGGCCG 150 G MYB::NFIB (M13N9) MYB13 F TCTTCTGCTCACACCACTGG NFIB9 R CGTAATTTTGGACATTGGCCG 160 SND1::BRAF (S9B9) SND1 F CGATTCACCTGTCCAGCATC BRAF R CGCTGAGGTCCTGGAGATTT 184 TBL1XR1::PIK3CA TBL1XR1 F TTTCCTTGTGCCTCCATTCC PIK3CA R GTCGTGGAGGCATTGTTCTG 195 (T1P2) TMPRSS2 TMPRSS2::ERG (T2E4) CGCGGCAGGTCATATTGAA ERG R CCTTCCCATCGATGTTCTGG 190 F WHSC1L1::FGFR1 WHSC1L1 TGATCGCACTGACACGGC FGFR1 R ACAAGGCTCCACATCTCCAT 108 (W1F2) F Table S2. Clinical and diagnostic implications of the detected gene fusions. Reclassification Entities Where Entity Where Fusion Is Based on Molecular Fusion Is Fusion Drug Ref. -

Crizotinib in ROS1-Positive Non-Small Cell Lung Cancer: an Asian Large Phase II Trial Confirms Its Key-Role
795 Editorial Crizotinib in ROS1-positive non-small cell lung cancer: an Asian large phase II trial confirms its key-role Paolo Maione, Cesare Gridelli Division of Medical Oncology, S. G. Moscati Hospital, Avellino, Italy Correspondence to: Cesare Gridelli. Division of Medical Oncology, S. G. Moscati Hospital, Contrada Amoretta, 83100 Avellino, Italy. Email: [email protected]. Comment on: Wu YL, Yang JC, Kim DW, et al. Phase II Study of Crizotinib in East Asian Patients With ROS1-Positive Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2018;36:1405-11. Submitted Jul 31, 2018. Accepted for publication Aug 06, 2018. doi: 10.21037/tcr.2018.08.13 View this article at: http://dx.doi.org/10.21037/tcr.2018.08.13 The ROS1 oncogene encodes an orphan receptor tyrosine rearrangements in NSCLC, ROS1 fusions confer sensitivity kinase related to anaplastic lymphoma kinase (ALK), along to the ALK/ROS1/MET inhibitor crizotinib (7). Moreover, with members of the insulin-receptor family (1). ROS1 in preclinical models, crizotinib seems more effective in rearrangement can be found in several human cancers ROS1-positive than in ALK-positive NSCLC. However, as cholangiocarcinoma, gastric cancer, ovarian cancer, crizotinib was originally developed as a MET inhibitor, and glioblastoma multiforme, and with therapeutic implications subsequently approved for ALK-positive NSCLC. A very in non-small cell lung cancer (NSCLC) (2-4). As a relevant phase I study named PROFILE 1001 enrolled also consequence of the rearrangement, a portion of ROS1, that 50 patients with advanced ROS1-positive NSCLC that were includes the entire tyrosine kinase domain, is fused with 1 of treated with crizotinib. -

Myeloid Innate Immunity Mouse Vapril2018
Official Symbol Accession Alias / Previous Symbol Official Full Name 2810417H13Rik NM_026515.2 p15(PAF), Pclaf RIKEN cDNA 2810417H13 gene 2900026A02Rik NM_172884.3 Gm449, LOC231620 RIKEN cDNA 2900026A02 gene Abcc8 NM_011510.3 SUR1, Sur, D930031B21Rik ATP-binding cassette, sub-family C (CFTR/MRP), member 8 Acad10 NM_028037.4 2410021P16Rik acyl-Coenzyme A dehydrogenase family, member 10 Acly NM_134037.2 A730098H14Rik ATP citrate lyase Acod1 NM_008392.1 Irg1 aconitate decarboxylase 1 Acot11 NM_025590.4 Thea, 2010309H15Rik, 1110020M10Rik,acyl-CoA Them1, thioesterase BFIT1 11 Acot3 NM_134246.3 PTE-Ia, Pte2a acyl-CoA thioesterase 3 Acox1 NM_015729.2 Acyl-CoA oxidase, AOX, D130055E20Rikacyl-Coenzyme A oxidase 1, palmitoyl Adam19 NM_009616.4 Mltnb a disintegrin and metallopeptidase domain 19 (meltrin beta) Adam8 NM_007403.2 CD156a, MS2, E430039A18Rik, CD156a disintegrin and metallopeptidase domain 8 Adamts1 NM_009621.4 ADAM-TS1, ADAMTS-1, METH-1, METH1a disintegrin-like and metallopeptidase (reprolysin type) with thrombospondin type 1 motif, 1 Adamts12 NM_175501.2 a disintegrin-like and metallopeptidase (reprolysin type) with thrombospondin type 1 motif, 12 Adamts14 NM_001081127.1 Adamts-14, TS14 a disintegrin-like and metallopeptidase (reprolysin type) with thrombospondin type 1 motif, 14 Adamts17 NM_001033877.4 AU023434 a disintegrin-like and metallopeptidase (reprolysin type) with thrombospondin type 1 motif, 17 Adamts2 NM_001277305.1 hPCPNI, ADAM-TS2, a disintegrin and ametalloproteinase disintegrin-like and with metallopeptidase thrombospondin -

Detection of Lung Adenocarcinoma with ROS1 Rearrangement by IHC, FISH, and RT-PCR and Analysis of Its Clinicopathologic Features
Journal name: OncoTargets and Therapy Article Designation: Original Research Year: 2016 Volume: 9 OncoTargets and Therapy Dovepress Running head verso: Cao et al Running head recto: Detection of lung adenocarcinoma with ROS1 rearrangement open access to scientific and medical research DOI: http://dx.doi.org/10.2147/OTT.S94997 Open Access Full Text Article ORIGINAL RESEARCH Detection of lung adenocarcinoma with ROS1 rearrangement by IHC, FISH, and RT-PCR and analysis of its clinicopathologic features Bing Cao1–3,* Objective: To detect ROS1 rearrangement using three different assays, including Ping Wei1–3,* immunohistochemistry (IHC), fluorescence in situ hybridization (FISH), and reverse transcription Zebing Liu4 polymerase chain reaction (RT-PCR), and to analyze the clinicopathologic features of ROS1 Rui Bi1–3 rearrangement in patients with lung adenocarcinoma. Yongming Lu1–3 Methods: One hundred eighty-three consecutive patients with lung adenocarcinoma with Ling Zhang1–3 operation and follow-up data were analyzed for ROS1 rearrangement by IHC, FISH, and RT- PCR. PCR products of the RT-PCR-positive samples were sequenced for confirmation of the Jing Zhang1–3 specific fusion partners. Yusi Yang1–3 Results: Three of the 183 (1.64%) cases were identified to be positive for ROS1 rearrange- 1–3 Chen Shen ment through all three methods. The fusion patterns were CD74 e6-ROS1 e32, CD74 e6-ROS1 1–3 Xiang Du e34, and TPM3 e8-ROS1 e35, respectively. FISH-positive cases showed two types of signals, 1–3 Xiaoyan Zhou single 3′ signals (green) and split red and green signals. Using FISH as a standard method, the 1Department of Pathology, Fudan sensitivity and specificity of ROS1 IHC with +1 staining or more were 100% and 96.67%, University Shanghai Cancer Center, respectively.