2 Ref. Ares(2019)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Verification of Vulnerable Zones Identified Under the Nitrate Directive \ and Sensitive Areas Identified Under the Urban Waste W
CONTENTS 1 INTRODUCTION 1 1.1 THE URBAN WASTEWATER TREATMENT DIRECTIVE (91/271/EEC) 1 1.2 THE NITRATES DIRECTIVE (91/676/EEC) 3 1.3 APPROACH AND METHODOLOGY 4 2 THE OFFICIAL GREEK DESIGNATION PROCESS 9 2.1 OVERVIEW OF THE CURRENT SITUATION IN GREECE 9 2.2 OFFICIAL DESIGNATION OF SENSITIVE AREAS 10 2.3 OFFICIAL DESIGNATION OF VULNERABLE ZONES 14 1 INTRODUCTION This report is a review of the areas designated as Sensitive Areas in conformity with the Urban Waste Water Treatment Directive 91/271/EEC and Vulnerable Zones in conformity with the Nitrates Directive 91/676/EEC in Greece. The review also includes suggestions for further areas that should be designated within the scope of these two Directives. Although the two Directives have different objectives, the areas designated as sensitive or vulnerable are reviewed simultaneously because of the similarities in the designation process. The investigations will focus upon: • Checking that those waters that should be identified according to either Directive have been; • in the case of the Nitrates Directive, assessing whether vulnerable zones have been designated correctly and comprehensively. The identification of vulnerable zones and sensitive areas in relation to the Nitrates Directive and Urban Waste Water Treatment Directive is carried out according to both common and specific criteria, as these are specified in the two Directives. 1.1 THE URBAN WASTEWATER TREATMENT DIRECTIVE (91/271/EEC) The Directive concerns the collection, treatment and discharge of urban wastewater as well as biodegradable wastewater from certain industrial sectors. The designation of sensitive areas is required by the Directive since, depending on the sensitivity of the receptor, treatment of a different level is necessary prior to discharge. -

Topo-Kalymnos.Pdf
MUNICIPAL TOURIST ORGANIZATION OF KALYMNOS. ( fin October 2005 ) KALYMNOS Rock climbing sectors & routes. LOCALISATION TO MOST OF MAIN SECTORS The white cubic pilars, along the main road, indicate the beginning of the paths to most of the climbing sectors. They also have useful information how to proceed the climbing area such as walking time and color used for marking the path. The stars * indicate the quality of the routes. * good ** very good *** excellent. USEFUL TELEPHONES. 22430 29301 Police 22430 24444 / 29304 Port Atithorities 22430 23025 / 166 Hospital 22430 50300 Taxi Station 22430 59056 Tourist Information. 22430 59445 Climbing înfo desk ATTENTION. Rock climbing on Kalymnos is under your own responsibility. Climbers are responsable for taking all necessary safety precautions. SECTEURS DE KALYMNOS ( Du nord au sud ) Styx Emborios SYKATI Kreissal SYKATI Thalassa 1- Baby house Palace Skalia pillar 2- Ghost kitchen 3- Cave 4- Nenuphar 5- Archi & balcon helvetic RINA Grey zone Sea breeze 6- Summer time 7- Kasteli (fort) 8- Odyssey 9- Ocean dream Iliada Muses 10- Jurassic park 11- Spartacus Afternoon 12- Grande grotta Panorama Kalydna Ianis Poets Zeus Gerakios Eros St constantine Telendos north Mystere Ouriana Symblegades Petres 13- Austrians Mantres 14- Monastery St Fotios Prophet Ilias Dodoni Sea Museum Paradise beach Rina Cross 1) STYX . Access : 100m to the left of « François Guillot ». Use the 4) SOPHIE . same path. Access : The same as for Kreissaal and a few minutes to the Routes : right, kastri (fort). 20min total time 1. Inuendo œ 6c 22m *** Routes : 2. Waiting for the sping œ 6b 22m *** 1. Project œ 30m 3. -

Tourism Development in Greek Insular and Coastal Areas: Sociocultural Changes and Crucial Policy Issues
Tourism Development in Greek Insular and Coastal Areas: Sociocultural Changes and Crucial Policy Issues Paris Tsartas University of the Aegean, Michalon 8, 82100 Chios, Greece The paperanalyses two issuesthat have characterised tourism development inGreek insularand coastalareas in theperiod 1970–2000. The firstissue concerns the socioeco- nomic and culturalchanges that have taken place in theseareas and ledto rapid– and usuallyunplanned –tourismdevelopment. The secondissue consists of thepolicies for tourismand tourismdevelopment atlocal,regional and nationallevel. The analysis focuseson therole of thefamily, social mobility issues,the social role of specific groups, and consequencesfor the manners, customs and traditionsof thelocal popula- tion.It also examines the views and reactionsof localcommunities regarding tourism and tourists.There is consideration of thenew productive structuresin theseareas, including thedowngrading of agriculture,the dependence of many economicsectors on tourism,and thelarge increase in multi-activityand theblack economy. Another focusis on thecharacteristics of masstourism, and on therelated problems and criti- cismsof currenttourism policies. These issues contributed to amodel of tourism development thatintegrates the productive, environmental and culturalcharacteristics of eachregion. Finally, the procedures and problemsencountered in sustainabledevel- opment programmes aiming at protecting the environment are considered. Social and Cultural Changes Brought About by Tourism Development in the Period 1970–2000 The analysishere focuseson three mainareas where these changesare observed:sociocultural life, productionand communication. It should be noted thata large proportionof all empirical studies of changesbrought aboutby tourism development in Greece have been of coastal and insular areas. Social and cultural changes in the social structure The mostsignificant of these changesconcern the family andits role in the new ‘urbanised’social structure, social mobility and the choicesof important groups, such as young people and women. -

Report on Territorial Diagnosis
CREADIS3: REPORT ON TERRITORIAL DIAGNOSIS. WESTERN GREECE Regional Development Fund on behalf of Region of Western Greece June 2018 2 INDEX 1. General Introduction ........................................................................................................... 3 1.1. The Project .......................................................................................................................3 1.2. The Region of Western Greece and the Project ........................................................4 2. Regional contexts ................................................................................................................ 5 2.1. Territory`s General Profile ..............................................................................................5 2.2. Territory`s CCI Profile ....................................................................................................8 3. CCI Sector Analysis: Evolution and Current Situation ................................................12 3.1. Evolution .........................................................................................................................12 3.2. Current Situation...........................................................................................................14 3.3. Creative Districs ............................................................................................................15 4. CCI Sector characterization .............................................................................................17 4.1. Stakeholders -
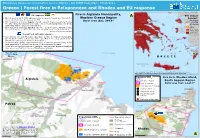
Greece | Forest Fires in Peloponnese and Rhodes and EU Response
Emergency Response Coordination Centre (ERCC) – DG ECHO Daily Map | 04/08/2021 Greece | Forest fires in Peloponnese and Rhodes and EU response Fire in Aigialeia Municipality, EU response A Fire danger ▪ On 3 August at 18:30 UTC, Greece made a request of assistance through the Western Greece Region forecast1 Union Civil Protection Mechanism (UCPM). Burnt area (ha): 394.9* 3-9 August ▪ On 4 August at 3:31 UTC, Cyprus offered 1 ground forest fire fighting module Source: JRC-EFFIS (40 pax) and 2 air tractors with 8 pax. The offer was accepted by the Greek Moderate authorities and the deployment is ongoing. ▪ On 4 August at 8:48 UTC, Sweden offered 1 aerial forest fire fighting module High A (2 fireboss via the rescEU). The offer was accepted by the Greek authorities. Very high Source: DG ECHO, as of 4 August Extreme Impact and national response ▪ According to the Civil Protection Operation Center, in Aigialeia Municipality, Aegean 230 firefighters with 116 vehicles were operating in the area, assisted by 5 Sea ground force groups, 8 helicopters and 8 planes. ▪ In Rhodes Municipality, 159 firefighters with 34 vehicles were operating in the area, assisted by 6 ground force group, 6 helicopters and 3 planes. ▪ There are no reported injuries or fatalities, and although damaged buildings have been reported there are no official figures available Source: Greek Civil Protection, as of 3 August at 18:36 UTC Approximately Gulf of Corinth 10 km to Patras B GREECE Sea of Crete MEDITERRANEAN SEA 1The maximum value of the fire danger forecast module of EFFIS, which generates daily maps of forecasted fire danger level using numerical weather predictions. -
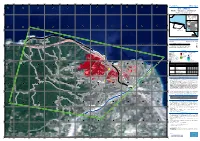
Grading - Overview Map 01 0 0 0 0 4 4 4 4 2 2
580000 581000 582000 583000 584000 585000 586000 587000 588000 589000 21°54'40"E 21°55'20"E 21°56'0"E 21°56'40"E 21°57'20"E 21°58'0"E 21°58'40"E 21°59'20"E 22°0'0"E 22°0'40"E 22°1'20"E N " 0 GLIDE number: N/A Activation ID: EMSR525 4 ' 0 2 ° N Int. Charter Act. ID: N/A Product N.: 01ZIRIA, v1 8 " 3 0 4 ' 0 2 ° 8 3 Ziria - GREECE Wildfire - Situation as of 02/08/2021 0 0 Grading - Overview map 01 0 0 0 0 4 4 4 4 2 2 4 4 Sterea Ellada Fokida ! ! ! ! ! ! ! ! Black ! ! Bulgaria ! ! ! ! Sea ! ! Ionian Sea ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! Albania ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! Greec e ! ! ! ! ! ! ! N ! ! ! ! ! Aegean ! ! ! ! " ! ! ! ! ! ! ! 0 ' ! ! Sea 0 ! ! 2 ! ! ! ! ! ° ! ! ! ! Ziria ! 8 ! N ! " 3 ! ! Turkey ! (! ! ! 0 ! ! ' ! ! ! ! ! ! ! 0 ! Athens ! Ionian ! ^ ! 2 ° ! ! Sea ! ! 8 ! ! 3 ! ! ! ! ! ! 0 0 ! ! Sea of ! ! 0 0 ! ! Achaia ! ! ! ! ! ! ! ! ! ! Crete 0 0 ! ! ! ! ! ! ! ! 3 3 ! ! ! ! 4 4 ! ! Mediterranean ! ! 2 2 ! ! Dytiki Ellada ! 4 4 ! Sea ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! 3 ! ! ! ! km ! ! ! ! ! ! ! ! ! ! Π αναγοπούλα ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! Cartographic Information ! ! ! ! ! ! ! ! ! ! ! ! ! ! Full color A1, 200 dpi resolution ! ! 1:15500 ! ! ! ! ! ! N ! ! " ! ! ! ! ! ! ! ! ! 0 ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! 2 ! ! 0 0.325 0.65 1.3 ' ! ! 0 0 ! ! ! ! ! 9 ! ! ! ! ! 0 0 1 ! ! ! ! ! ! ! ° ! ! ! ! ! km ! N ! ! ! ! 0 0 ! ! 8 " ! ! ! ! ! ! ! 2 2 3 ! 0 -

Cyanobacterial Toxins and Peptides in Lake Vegoritis, Greece
toxins Article Cyanobacterial Toxins and Peptides in Lake Vegoritis, Greece Sevasti-Kiriaki Zervou 1 , Kimon Moschandreou 2, Aikaterina Paraskevopoulou 1, Christophoros Christophoridis 1 , Elpida Grigoriadou 3, Triantafyllos Kaloudis 1, Theodoros M. Triantis 1 , Vasiliki Tsiaoussi 2 and Anastasia Hiskia 1,* 1 Laboratory of Photo-Catalytic Processes and Environmental Chemistry, Institute of Nanoscience & Nanotechnology, National Center for Scientific Research “Demokritos”, Patriarchou Grigoriou E & 27 Neapoleos Str, 15310 Agia Paraskevi, Athens, Greece; [email protected] (S.-K.Z.); [email protected] (A.P.); [email protected] (C.C.);[email protected] (T.K.); [email protected] (T.M.T.) 2 The Goulandris Natural History Museum—Greek Biotope/Wetland Centre, 14th km Thessaloniki-Mihaniona, Thermi P.O. Box 60394, 57001 Thessaloniki, Greece; [email protected] (K.M.); [email protected] (V.T.) 3 Water Resources Management Agency of West Macedonia, 50100 Kozani, Decentralized Administration of Epirus—Western Macedonia, Greece; [email protected] * Correspondence: [email protected] Abstract: Cyanotoxins (CTs) produced by cyanobacteria in surface freshwater are a major threat for public health and aquatic ecosystems. Cyanobacteria can also produce a wide variety of other understudied bioactive metabolites such as oligopeptides microginins (MGs), aeruginosins (AERs), aeruginosamides (AEGs) and anabaenopeptins (APs). This study reports on the co-occurrence of CTs and cyanopeptides (CPs) in Lake Vegoritis, Greece and presents their variant-specific profiles obtained during 3-years of monitoring (2018–2020). Fifteen CTs (cylindrospermopsin (CYN), anatoxin (ATX), nodularin (NOD), and 12 microcystins (MCs)) and ten CPs (3 APs, 4 MGs, 2 AERs and aeruginosamide Citation: Zervou, S.-K.; (AEG A)) were targeted using an extended and validated LC-MS/MS protocol for the simultaneous Moschandreou, K.; Paraskevopoulou, determination of multi-class CTs and CPs. -

Ganas, A., Serpelloni, E., Drakatos, G., Kolligri, M., Adamis, I., Tsimi, Ch
This article was downloaded by: [HEAL-Link Consortium] On: 20 October 2009 Access details: Access Details: [subscription number 772810551] Publisher Taylor & Francis Informa Ltd Registered in England and Wales Registered Number: 1072954 Registered office: Mortimer House, 37-41 Mortimer Street, London W1T 3JH, UK Journal of Earthquake Engineering Publication details, including instructions for authors and subscription information: http://www.informaworld.com/smpp/title~content=t741771161 The Mw 6.4 SW-Achaia (Western Greece) Earthquake of 8 June 2008: Seismological, Field, GPS Observations, and Stress Modeling A. Ganas a; E. Serpelloni b; G. Drakatos a; M. Kolligri a; I. Adamis a; Ch. Tsimi a; E. Batsi a a Institute of Geodynamics, National Observatory of Athens, Athens, Greece b Istituto Nazionale di Geofisica e Vulcanologia, Centro Nazionale Terremoti, Bologna, Italy Online Publication Date: 01 December 2009 To cite this Article Ganas, A., Serpelloni, E., Drakatos, G., Kolligri, M., Adamis, I., Tsimi, Ch. and Batsi, E.(2009)'The Mw 6.4 SW- Achaia (Western Greece) Earthquake of 8 June 2008: Seismological, Field, GPS Observations, and Stress Modeling',Journal of Earthquake Engineering,13:8,1101 — 1124 To link to this Article: DOI: 10.1080/13632460902933899 URL: http://dx.doi.org/10.1080/13632460902933899 PLEASE SCROLL DOWN FOR ARTICLE Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf This article may be used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden. The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. -

Contemporary Kinematics of the South Aegean Area (Greece) Detected with Continuous GNSS Measurements
EGU2020-7656, updated on 03 Oct 2021 https://doi.org/10.5194/egusphere-egu2020-7656 EGU General Assembly 2020 © Author(s) 2021. This work is distributed under the Creative Commons Attribution 4.0 License. Contemporary Kinematics of the South Aegean Area (Greece) Detected with Continuous GNSS Measurements Vassilis Sakkas, Chrysa Doxa, Andreas Tzanis, and Haralambos Kranis National & Kapodistrian University of Athens, Department of Geology and the Geoenvironment, Athens, Greece ([email protected]) We examine the kinematic characteristics of the crustal deformation in the broader southern Aegean region using 47 permanent GNNS stations distributed across the eastern Peloponnesus, Attica, Cyclades, Dodecanese, Crete and the coast of western Anatolia. Our analysis is based on the study of velocity vectors relative to local reference points at the western and eastern halves of the study area, as well as on the strain field calculated from absolute velocity vectors across the study area. We demonstrate that the South Aegean region undergoes complex distributed block deformation. At the eastern end of the study area this varies from N210°-N220° extension and with crustal thinning across NE Peloponnesus – Attica, to N210°-N220° compression between the central- eastern Peloponnesus and western Crete, both consistent with the geodynamic setting of the Hellenic Subduction System. A principal feature of the S. Aegean crust appears to be a broad shear zone extending between the islands of Samos/Ikaria and Kalymnos, Paros/Naxos and Amorgos and Milos – Santorini; It exhibits left-lateral kinematics and its southern boundary appears to coincide with the Amorgos – Santorini ridge and comprise the Anhydros basin and associated volcanic field (including Columbo and Santorini). -

Ancient Greece
αρχαία Ελλάδα (Ancient Greece) The Birthplace of Western Civilization Marshall High School Mr. Cline Western Civilization I: Ancient Foundations Unit Three AA * European Civilization • Neolithic Europe • Europe’s earliest farming communities developed in Greece and the Balkans around 6500 B.C. • Their staple crops of emmer wheat and barley were of near eastern origin, indicating that farming was introduced by settlers from Anatolia • Farming spread most rapidly through Mediterranean Europe. • Society was mostly composed of small, loose knit, extended family units or clans • They marked their territory through the construction of megalithic tombs and astronomical markers • Stonehenge in England • Hanobukten, Sweden * European Civilization • Neolithic Europe • Society was mostly composed of small, loose knit, extended family units or clans • These were usually built over several seasons on a part time basis, and required little organization • However, larger monuments such as Stonehenge are evidence of larger, more complex societies requiring the civic organization of a territorial chiefdom that could command labor and resources over a wide area. • Yet, even these relatively complex societies had no towns or cities, and were not literate * European Civilization • Ancient Aegean Civilization • Minos and the Minotaur. Helen of Troy. Odysseus and his Odyssey. These names, still famous today, bring to mind the glories of the Bronze Age Aegean. • But what was the truth behind these legends? • The Wine Dark Sea • In Greek Epic, the sea was always described as “wine dark”, a common appellation used by many Indo European peoples and languages. • It is even speculated that the color blue was not known at this time. Not because they could not see it, but because their society just had no word for it! • The Aegean Sea is the body of water which lays to the east of Greece, west of Turkey, and north of the island of Crete. -

Quick Ferry Guide 2018 - Correct As at 17 April
Quick Ferry Guide 2018 - correct as at 17 April Times 4 September to 30 September 2018As always subject to alteration at short notice - always check with operators Rhodes NORTH Date restrictions dep Port Area Panormitis Arr Symi Arr Kos Arr Ferry Operator Comment Monday 08:30 Kolonna 09:20 10:55 Dodekanisos Express/Pride Dodekanisos Seaways Continues to Kalymnos, Leros, Lipsi and Patmos 09:00 Akandia 11:00 Symi Sea Dreams 09:30 Akandia 12:30 13:00 Panagia Skiadeni Dodekanisos Seaways Tuesday 09:00 Akandia 11:00 Symi Sea Dreams 09:30 Akandia 11:00 Panagia Skiadeni Dodekanisos Seaways Wednesday 08:30 Kolonna 09:20 10:55 Dodekanisos Express/Pride Dodekanisos Seaways Continues to Kalymnos, Leros, Lipsi and Agathonisi 09:00 Akandia 11:00 Symi Sea Dreams 09:30 Akandia 12:30 13:00 Panagia Skiadeni Dodekanisos Seaways Until 19 Sept 13:00 Kolonna 13:50 15:20 Dodekanisos Express/Pride Dodekanisos Seaways Continues to Leros, Patmos, and Samos. 18:00 Akandia 19:15 21:45 Blue Star Patmos Blue Star Continues to Kalymnos, Leros, Lipsi, Patmos & Piraeus Thursday 26 Sept only 08:30 Kolonna 09:25 10:55 Dodekanisos Express/Pride Dodekanisos Seaways Continues to Kalymnos, Leros, Lipsi, Patmos, Samos 09:00 Akandia 11:00 Symi Sea Dreams 09:30 Akandia 11:00 Panagia Skiadeni Dodekanisos Seaways Friday 08:30 Kolonna 09:20 10:55 Dodekanisos Express/Pride Dodekanisos Seaways Continues to Kalymnos, Leros, Lipsi & Patmos 09:00 Akandia 11:00 Symi Sea Dreams 09:30 Akandia 12:30 13:00 Panagia Skiadeni Dodekanisos Seaways 19:00 Akandia 20:30 01:50 Blue Star Patmos Blue -

Iconography of the Gorgons on Temple Decoration in Sicily and Western Greece
ICONOGRAPHY OF THE GORGONS ON TEMPLE DECORATION IN SICILY AND WESTERN GREECE By Katrina Marie Heller Submitted to the Faculty of The Archaeological Studies Program Department of Sociology and Archaeology In partial fulfillment of the requirements for the degree of Bachelor of Science University of Wisconsin-La Crosse 2010 Copyright 2010 by Katrina Marie Heller All Rights Reserved ii ICONOGRAPHY OF THE GORGONS ON TEMPLE DECORATION IN SICILY AND WESTERN GREECE Katrina Marie Heller, B.S. University of Wisconsin - La Crosse, 2010 This paper provides a concise analysis of the Gorgon image as it has been featured on temples throughout the Greek world. The Gorgons, also known as Medusa and her two sisters, were common decorative motifs on temples beginning in the eighth century B.C. and reaching their peak of popularity in the sixth century B.C. Their image has been found to decorate various parts of the temple across Sicily, Southern Italy, Crete, and the Greek mainland. By analyzing the city in which the image was found, where on the temple the Gorgon was depicted, as well as stylistic variations, significant differences in these images were identified. While many of the Gorgon icons were used simply as decoration, others, such as those used as antefixes or in pediments may have been utilized as apotropaic devices to ward off evil. iii Acknowledgements I would first like to thank my family and friends for all of their encouragement throughout this project. A special thanks to my parents, Kathy and Gary Heller, who constantly support me in all I do. I need to thank Dr Jim Theler and Dr Christine Hippert for all of the assistance they have provided over the past year, not only for this project but also for their help and interest in my academic future.