Anisoptera: Cordulegastridae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Geographical Variation in the Wing Morphology of the Golden-Ringed
Bull. Natl. Mus. Nat. Sci., Ser. A, 38(2), pp. 65–73, May 22, 2012 Geographical Variation in the Wing Morphology of the Golden-ringed Dragonfly Anotogaster sieboldii (Selys, 1854) (Odonata, Cordulegastridae) Detected by Landmark-based Geometric Morphometrics Takuya Kiyoshi1 and Tsutomu Hikida2 1 Department of Zoology, National Museum of Nature and Science, 4–1–1 Amakubo, Tsukuba, Ibaraki, 305–0005 Japan E-mail: [email protected] 2 Department of Zoology, Graduate School of Science, Kyoto University, Kitashirakawaoiwakecho, Sakyo-ku, Kyoto, Kyoto, 606–8502 Japan (Received 18 January 2012; accepted 4 April 2012) Abstract A previous molecular phylogenetic study showed that Anotogaster sieboldii has at least 6 monophyletic groups that are allopatrically distributed in the northern area of Asia (Japanese main islands and Korean Peninsula), Amamioshima, Okinawajima, Yaeyama islands, Taiwan and East China. These groups are difficult to distinguish by qualitative genital morphology; however, canonical variate analysis and linear discriminant analysis of the hind wing shape have been used to clearly distinguish them from each other. In the present study, multiple comparisons of the lengths of the abdomen and hind wing showed that these lengths did not differ significantly among the groups. In particular, the variation in the size range, in the lineage from the northern area, widely overlapped that of the lineages from the other areas. In evaluating the morphological differ- ences, the wing shape was found to be more sensitive than other size variables. Key words : geometric morphometrics, shape, Odonata. Introduction groups (Askew, 2004). Anotogaster sieboldii (Selys, 1854) is a large The family Cordulegastridae of the order Odo- cordulegastrid dragonfly that is distributed in nata consists of 40 species belonging to 5 genera Insular East Asia, Korean Peninsula and East (Tsuda, 2000). -

Using Specimens from the Past to Understand the Living World Through Digitization
Using specimens from the past to understand the living world through digitization Drs. Jessica L. Ware, William Kuhn, Dirk Gassmann and John Abbott Rutgers University, Newark Dragonflies: Order Odonata Suborders Anisoptera (unequal wings): Dragonflies ~3000 species Anisozygoptera ~3 species Zygoptera: Damselflies ~3000 species Perchers, Fliers, Migrators, & Homebodies Dragonfly flight Wing venation affects wing camber, lift, and ultimately flight patterns Dragonfly flight Stiffness varies along length of the wing with vein density and thickness Dragonfly flight Certain wing traits are correlated with specific flight styles Dragonfly collections: invaluable treasures Collection name # spp. #specimens Florida State Collection 2728 150K Ware Lab Collection 373 4K Smithsonian Collection 253 200K M.L. May Collection 300 10K Dragonfly collections: invaluable treasures Harness information in collections Targeted Odonata Wing Digitization (TOWD) project TOWD project scanning protocol TOWD project scanning protocol TOWD project TOWD project TOWD project 10 10 TOWD project More information at: https://willkuhn.github.io/towd/ Aspect ratios: How elongate is the wing compared to its overall area? • High Aspect ratio Low Aspect ratio Long narrow wings, Wing Short broad wings, optimized for: Wing optimized long-distance flight for: Maneuverability, turning High Aspect Low Aspect Ratio Ratio More data on aspect ratios, better interpretations? 2007: Hand measured forewings of 85 specimens, 7 months work More data on apsect ratios, better interpretations? 2007: Hand measured forewings of 85 specimens, 7 months 2019: Odomatic measurements for 206 work specimens, 2-3 minutes of work! Are there differences in aspect ratios? Perchers have significantly lower aspect ratios than fliers. The p-value is .001819. The result is significant at p < .05. -

The Impacts of Urbanisation on the Ecology and Evolution of Dragonflies and Damselflies (Insecta: Odonata)
The impacts of urbanisation on the ecology and evolution of dragonflies and damselflies (Insecta: Odonata) Giovanna de Jesús Villalobos Jiménez Submitted in accordance with the requirements for the degree of Doctor of Philosophy (Ph.D.) The University of Leeds School of Biology September 2017 The candidate confirms that the work submitted is her own, except where work which has formed part of jointly-authored publications has been included. The contribution of the candidate and the other authors to this work has been explicitly indicated below. The candidate confirms that appropriate credit has been given within the thesis where reference has been made to the work of others. The work in Chapter 1 of the thesis has appeared in publication as follows: Villalobos-Jiménez, G., Dunn, A.M. & Hassall, C., 2016. Dragonflies and damselflies (Odonata) in urban ecosystems: a review. Eur J Entomol, 113(1): 217–232. I was responsible for the collection and analysis of the data with advice from co- authors, and was solely responsible for the literature review, interpretation of the results, and for writing the manuscript. All co-authors provided comments on draft manuscripts. The work in Chapter 2 of the thesis has appeared in publication as follows: Villalobos-Jiménez, G. & Hassall, C., 2017. Effects of the urban heat island on the phenology of Odonata in London, UK. International Journal of Biometeorology, 61(7): 1337–1346. I was responsible for the data analysis, interpretation of results, and for writing and structuring the manuscript. Data was provided by the British Dragonfly Society (BDS). The co-author provided advice on the data analysis, and also provided comments on draft manuscripts. -

Dragonf Lies and Damself Lies of Europe
Dragonf lies and Damself lies of Europe A scientific approach to the identification of European Odonata without capture A simple yet detailed guide suitable both for beginners and more expert readers who wish to improve their knowledge of the order Odonata. This book contains images and photographs of all the European species having a stable population, with chapters about their anatomy, biology, behaviour, distribution range and period of flight, plus basic information about the vagrants with only a few sightings reported. On the whole, 143 reported species and over lies of Europe lies and Damself Dragonf 600 photographs are included. Published by WBA Project Srl CARLO GALLIANI, ROBERTO SCHERINI, ALIDA PIGLIA © 2017 Verona - Italy WBA Books ISSN 1973-7815 ISBN 97888903323-6-4 Supporting Institutions CONTENTS Preface 5 © WBA Project - Verona (Italy) Odonates: an introduction to the order 6 WBA HANDBOOKS 7 Dragonflies and Damselflies of Europe Systematics 7 ISSN 1973-7815 Anatomy of Odonates 9 ISBN 97888903323-6-4 Biology 14 Editorial Board: Ludivina Barrientos-Lozano, Ciudad Victoria (Mexico), Achille Casale, Sassari Mating and oviposition 23 (Italy), Mauro Daccordi, Verona (Italy), Pier Mauro Giachino, Torino (Italy), Laura Guidolin, Oviposition 34 Padova (Italy), Roy Kleukers, Leiden (Holland), Bruno Massa, Palermo (Italy), Giovanni Onore, Quito (Ecuador), Giuseppe Bartolomeo Osella, l’Aquila (Italy), Stewart B. Peck, Ottawa (Cana- Predators and preys 41 da), Fidel Alejandro Roig, Mendoza (Argentina), Jose Maria Salgado Costas, Leon (Spain), Fabio Pathogens and parasites 45 Stoch, Roma (Italy), Mauro Tretiach, Trieste (Italy), Dante Vailati, Brescia (Italy). Dichromism, androchromy and secondary homochromy 47 Editor-in-chief: Pier Mauro Giachino Particular situations in the daily life of a dragonfly 48 Managing Editor: Gianfranco Caoduro Warming up the wings 50 Translation: Alida Piglia Text revision: Michael L. -

An Overview of Molecular Odonate Studies, and Our Evolutionary Understanding of Dragonfly and Damselfly (Insecta: Odonata) Behavior
International Journal of Odonatology Vol. 14, No. 2, June 2011, 137–147 Dragons fly, biologists classify: an overview of molecular odonate studies, and our evolutionary understanding of dragonfly and damselfly (Insecta: Odonata) behavior Elizabeth F. Ballare* and Jessica L. Ware Department of Biological Sciences, Rutgers, The State University of New Jersey, 195 University Ave., Boyden Hall, Newark, NJ, 07102, USA (Received 18 November 2010; final version received 3 April 2011) Among insects, perhaps the most appreciated are those that are esthetically pleasing: few capture the interest of the public as much as vibrantly colored dragonflies and damselflies (Insecta: Odonata). These remarkable insects are also extensively studied. Here, we review the history of odonate systematics, with an emphasis on discrepancies among studies. Over the past century, relationships among Odonata have been reinterpreted many times, using a variety of data from wing vein morphology to DNA. Despite years of study, there has been little consensus about odonate taxonomy. In this review, we compare odonate molecular phylogenetic studies with respect to gene and model selection, optimality criterion, and dataset completeness. These differences are discussed in relation to the evolution of dragonfly behavior. Keywords: Odonata; mitochondrion; nuclear; phylogeny; systematic; dragonfly; damselfly Introduction Why study Odonata? The order Odonata comprises three suborders: Anisozygoptera, Anisoptera, and Zygoptera. There are approximately 6000 species of Odonata described worldwide (Ardila-Garcia & Gregory, 2009). Of the three suborders Anisoptera and Zygoptera are by far the most commonly observed and collected, because there are only two known species of Anisozygoptera under the genus Epiophlebia. All odonate nymphs are aquatic, with a few rare exceptions such as the semi-aquatic Pseudocordulia (Watson, 1983), and adults are usually found near freshwater ponds, marshes, rivers (von Ellenrieder, 2010), streams, and lakes (although some species occur in areas of mild salinity; Corbet, 1999). -

© 2016 David Paul Moskowitz ALL RIGHTS RESERVED
© 2016 David Paul Moskowitz ALL RIGHTS RESERVED THE LIFE HISTORY, BEHAVIOR AND CONSERVATION OF THE TIGER SPIKETAIL DRAGONFLY (CORDULEGASTER ERRONEA HAGEN) IN NEW JERSEY By DAVID P. MOSKOWITZ A dissertation submitted to the Graduate School-New Brunswick Rutgers, The State University of New Jersey In partial fulfillment of the requirements For the degree of Doctor of Philosophy Graduate Program in Entomology Written under the direction of Dr. Michael L. May And approved by _____________________________________ _____________________________________ _____________________________________ _____________________________________ New Brunswick, New Jersey January, 2016 ABSTRACT OF THE DISSERTATION THE LIFE HISTORY, BEHAVIOR AND CONSERVATION OF THE TIGER SPIKETAIL DRAGONFLY (CORDULEGASTER ERRONEA HAGEN) IN NEW JERSEY by DAVID PAUL MOSKOWITZ Dissertation Director: Dr. Michael L. May This dissertation explores the life history and behavior of the Tiger Spiketail dragonfly (Cordulegaster erronea Hagen) and provides recommendations for the conservation of the species. Like most species in the genus Cordulegaster and the family Cordulegastridae, the Tiger Spiketail is geographically restricted, patchily distributed with its range, and a habitat specialist in habitats susceptible to disturbance. Most Cordulegastridae species are also of conservation concern and the Tiger Spiketail is no exception. However, many aspects of the life history of the Tiger Spiketail and many other Cordulegastridae are poorly understood, complicating conservation strategies. In this dissertation, I report the results of my research on the Tiger Spiketail in New Jersey. The research to investigate life history and behavior included: larval and exuvial sampling; radio- telemetry studies; marking-resighting studies; habitat analyses; observations of ovipositing females and patrolling males, and the presentation of models and insects to patrolling males. -

Indiana County Natural Heritage Inventory
Indiana County Natural Heritage Inventory Update 2021 Indiana County Natural Heritage Inventory 2021 Update Anna Johnson and Christopher Tracey, editors Prepared for: Southwest Pennsylvania Commission 112 Washington Pl #500 Pittsburgh, PA 15219 Prepared by: Pennsylvania Natural Heritage Program 800 Waterfront Drive Pittsburgh, PA 15222 Please cite this Natural Heritage Inventory report as: Johnson, Anna and Christopher Tracey, editors. 2021. Indiana County Natural Heritage Inventory. Pennsylvania Natural Heritage Program. Pittsburgh, PA. 1 ACKNOWLEDGEMENTS We would like to acknowledge the citizens and landowners of Indiana County and surrounding areas who volunteered infor- mation, time, and effort to the inventory and granted permission to access land. A big thank you goes to those who suggested areas of interest, provided data, and assisted with field surveys. Additional thanks goes to Ryan Gordon of the Southwest Pennsylvania Commission for providing support for this project. Advisory Committee to the 2021 update to the Indiana County Natural Heritage Inventory: • Byron Stauffer Jr. — Indiana County Executive Director of Planning and Development • Jeff Raykes — Indiana County Planner • Josh Krug — Indiana County Planner We want to recognize the Pennsylvania Natural Heritage Program and NatureServe for providing the foundation for the work that we perform for these studies. Current and former PNHP staff that contributed to this report includes JoAnn Albert, Jaci Braund, Charlie Eichelberger, Kierstin Carlson, Mary Ann Furedi, Steve Grund, Amy Jewitt, Anna Johnson, Susan Klugman, John Kunsman, Betsy Leppo, Jessica McPherson, Molly Moore, Ryan Miller, Greg Podniesinski, Megan Pulver, Erika Schoen, Scott Schuette, Emily Szoszorek, Kent Taylor, Christopher Tracey, Natalie Virbitsky, Jeff Wagner, Denise Watts, Joe Wisgo, Pete Woods, David Yeany, and Ephraim Zimmerman. -

DAMSELFLIES and DRAGONFLIES of SOUTHERN NEW YORK Basic
DAMSELFLIES AND DRAGONFLIES OF SOUTHERN NEW YORK A Beginner’s Guide The insect order Odonata is composed of two groups, the Damselflies (Zygoptera) and Dragonflies (Anisoptera). In North America there are approximately 122 species of damselflies and about 309 species of dragonflies. Of these, about 55 damselflies and 128 dragonflies occur in southern New York. Distinguishing damselflies from dragonflies is relatively easy: Damselflies – Front and hind wings similar in size and shape; eyes separated by more than their own width; at rest, wings meeting above the body or only partly expanded. Generally with weak, fluttery flight. Dragonflies – Front and hind wings dissimilar in size and shape, the hind wing considerably wider at base than the front wing; eyes meeting middorsally or not separated by a space greater than their own width; at rest, wings held horizontally. Generally strong fliers. Basic Anatomy Dragonfly Damselfly DAMSELFLIES Broad-winged Damsels (Calopterygidae) Wings held together at rest Wings with black, brown, amber or red markings Wings broad shaped without narrow stalk at base 8 species (2 genera) in North America Spreadwings (Lestidae) Wings spread at rest Wings clear or only slightly tinted Wings with narrow stalk at base Stigma long, length more than twice width 1 species (2 genera) in North America Pond Damsels (Coenargrionidae) Wings typically held together at rest Wings clear or only slightly yinted Wings with narrow stalk at base Stigmas short, length about equal to width 96 species (13 genera) in North America Guide to the Genera of Common Pond Damsels of Southern New York Pond Damsels are the most numerous and among the most difficult to identify of the Damselflies. -

The Classification and Diversity of Dragonflies and Damselflies (Odonata)*
Zootaxa 3703 (1): 036–045 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Correspondence ZOOTAXA Copyright © 2013 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3703.1.9 http://zoobank.org/urn:lsid:zoobank.org:pub:9F5D2E03-6ABE-4425-9713-99888C0C8690 The classification and diversity of dragonflies and damselflies (Odonata)* KLAAS-DOUWE B. DIJKSTRA1, GÜNTER BECHLY2, SETH M. BYBEE3, RORY A. DOW1, HENRI J. DUMONT4, GÜNTHER FLECK5, ROSSER W. GARRISON6, MATTI HÄMÄLÄINEN1, VINCENT J. KALKMAN1, HARUKI KARUBE7, MICHAEL L. MAY8, ALBERT G. ORR9, DENNIS R. PAULSON10, ANDREW C. REHN11, GÜNTHER THEISCHINGER12, JOHN W.H. TRUEMAN13, JAN VAN TOL1, NATALIA VON ELLENRIEDER6 & JESSICA WARE14 1Naturalis Biodiversity Centre, PO Box 9517, NL-2300 RA Leiden, The Netherlands. E-mail: [email protected]; [email protected]; [email protected]; [email protected]; [email protected] 2Staatliches Museum für Naturkunde Stuttgart, Rosenstein 1, 70191 Stuttgart, Germany. E-mail: [email protected] 3Department of Biology, Brigham Young University, 401 WIDB, Provo, UT. 84602 USA. E-mail: [email protected] 4Department of Biology, Ghent University, Ledeganckstraat 35, B-9000 Ghent, Belgium. E-mail: [email protected] 5France. E-mail: [email protected] 6Plant Pest Diagnostics Branch, California Department of Food & Agriculture, 3294 Meadowview Road, Sacramento, CA 95832- 1448, USA. E-mail: [email protected]; [email protected] 7Kanagawa Prefectural Museum of Natural History, 499 Iryuda, Odawara, Kanagawa, 250-0031 Japan. E-mail: [email protected] 8Department of Entomology, Rutgers University, Blake Hall, 93 Lipman Drive, New Brunswick, New Jersey 08901, USA. -

INTRODUCTION to Dragonfly and Damselfly Watching
Booklet.qxd 11.07.2003 10:59 AM Page 1 TEXAS PARKS AND WILDLIFE INTRODUCTION TO Dragonfly and Damselfly Watching BY MARK KLYM AND MIKE QUINN Booklet.qxd 11.07.2003 10:59 AM Page 2 Cover illustration by Rob Fleming. Booklet.qxd 11.07.2003 10:59 AM Page 3 Introduction to Dragonfly and Damselfly Watching By Mark Klym and Mike Quinn Acknowledgement This work would not have been possible without the input of Bob Behrstock, John Abbott and Sid Dunkle who provided technical information on the Order Odonata in Texas. This is not the first book about this order of insects, and the work of Sid Dunkle in Dragonflies Through Binoculars was a great help in assembling and presenting the material. Pat Morton was a great help in reviewing the material and keeping the work on track. Booklet.qxd 11.07.2003 10:59 AM Page 4 INTRODUCTION Background Dragonflies and Damselflies are members of the insect order Odonata, derived from the Greek word odonto meaning tooth. They are insects meaning that they have three body regions — a head, a thorax to which their four wings and six legs are attached and an abdomen. They are characterized by two pairs of net-veined wings and large compound eyes. Their wings are not linked together, allowing each wing to operate independently of the others. Damselflies have narrowly rectangular heads and eyes separated by more than their own width while dragonfly eyes are never separated by more than their own width. Both are preda- tors throughout their lives and valuable in destroying mosquitoes, gnats and other insects though they can become pests near beehives and may take other beneficial insects like butterflies. -
Dragonfly (Pg. 3-4) Head Eye Color
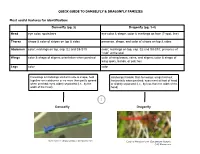
QUICK GUIDE TO DAMSELFLY & DRAGONFLY FAMILIES Most useful features for identification: Damselfly (pg. 2) Dragonfly (pg. 3-4) Head eye color; spots/bars eye color & shape; color & markings on face (T-spot, line) Thorax shape & color of stripes on top & sides presence, shape, and color of stripes on top & sides Abdomen color; markings on top, esp. S2 and S8-S10 color; markings on top, esp. S2 and S8-S10; presence of “club” at the end Wings color & shape of stigma; orientation when perched color of wing bases, veins, and stigma; color & shape of wing spots, bands, or patches Legs color color Forewings & hindwings similar in size & shape, held Hindwings broader than forewings; wings held out together over abdomen or no more than partly spread horizontally when perched; eyes meet at front of head when perched; eyes widely separated (i.e., by the or slightly separated (i.e., by less than the width of the width of the head) head) 1 Damselfly Dragonfly Vivid Dancer (Argia vivida); CAS Mazzacano Cardinal Meadowhawk (Sympetrum illotum); CAS Mazzacano DAMSELFLIES Wings narrow, stalked at base 2 Wings broad, colored, not stalked at base 3 Wings held askew Wings held together Broad-winged Damselfly when perched when perched (Calopterygidae); streams Spreadwing Pond Damsels (Coenagrionidae); (Lestidae); ponds ponds, streams Dancer (Argia); Wings held above abdomen; vivid colors streams 4 Wings held along Bluet (Enallagma); River Jewelwing (Calopteryx aequabilis); abdomen; mostly blue ponds CAS Mazzacano Dark abdomen with blue Forktail (Ischnura); tip; -

Check List of First Recorded Dragonfly (Odonata: Anisoptera) Fauna of District Lower Dir, Khyber Pakhtunkhwa, Pakistan
Arthropods, 2014, 3(2): 120-126 Article Check list of first recorded dragonfly (Odonata: Anisoptera) fauna of District Lower Dir, Khyber Pakhtunkhwa, Pakistan Farzana Perveen1, Anzela Khan2, Sayed Abdul Rauf3 1Departments of Zoology, Shaheed Benazir Bhutto University (SBBU), Main Campus, Sheringal, Khyber Pakhtunkhwa, Pakistan 2Beaconhouse School System, Margalla Campus (BMI-G), H-8, Islamabad, Pakistan 3Departments of Zoology, Shaheed Benazir Bhutto University (SBBU), Main Campus, Sheringal, Khyber Pakhtunkhwa, Pakistan E-mail: [email protected] Received 5 March 2014; Accepted 10 April 2014; Published online 1 June 2014 Abstract The dragonflies (Odonata: Anisoptera) are large, intermediate to small size, having different colours and variable morphological characters. They also carry ornamental and environmental indicator values. The first recorded, the collection of 318 dragonflies was made during May-July 2011 from district Lower Dir, Khyber Pakhtunkhwa, Pakistan. Among them 11 species of dragonflies were identified belonging to 3 families. The golden-ringed, Cordulegaster brevistigma brevistigma Selys is belonging to family Cordulegasteridae and Clubtails, Onychogomphus bistrigatus Selys is belonging to family Gomophidaed. The spine-legged redbolt, Rhodothemis rufa (Rambur); black-tailed skimmer, Orthetrum cancellatum Linnaeus; blue or black-percher, Diplacodes lefebvrei (Ramber); ground-skimmer, Diplacodes trivialis Rambur; common red-skimmer, Orthetrum pruinosum neglectum (Rambur); triangle-skimmer, Orthetrum triangulare triangulare