In Memoriam – Alan Hall Keith Burridge*
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Research Development Innovation 2013/2014 Report
Centro Nacional de Biotecnología Campus de Cantoblanco RESEARCH Darwin 3, Madrid 28049, Spain Tel.: [+ 34] 91 585 4500 / Fax: [+ 34] 91 585 4506 DEVELOPMENT www.cnb.csic.es INNOVATION 2013/2014 REPORT INDEX Welcome to the CNB ................................................................................................................................................. 9 Carmen Castresana 1 / Plant Molecular Genetics 13 Genetic and molecular basis of naturally-occurring variation in plant development .............................................. 14 Carlos Alonso-Blanco Plant immunity strategies against microbial pathogen infection .............................................................................. 15 Carmen Castresana Genetic control of shoot branching patterns in plants ............................................................................................. 16 Pilar Cubas Plant-pathogen interaction in viral infections ........................................................................................................... 17 Juan Antonio García / Carmen Simón Genes involved in root architecture and in arsenic phytoremediation .................................................................... 18 Antonio Leyva Tejada Regulation of gene activity in plants: the phosphate starvation rescue system ...................................................... 19 Javier Paz-Ares Light signalling and day length control of potato tuber formation ........................................................................... 20 Salomé -
BSCB Newsletter 2017D
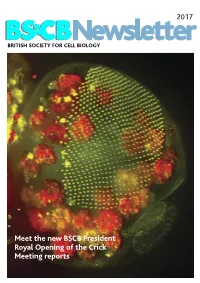
2017 BSCB Newsletter BRITISH SOCIETY FOR CELL BIOLOGY Meet the new BSCB President Royal Opening of the Crick Meeting reports 2017 CONTENTS BSCB Newsletter News 2 Book reviews 7 Features 8 Meeting Reports 24 Summer students 30 Society Business 33 Editorial Welcome to the 2017 BSCB newsletter. After several meeting hosted several well received events for our Front cover: years of excellent service, Kate Nobes has stepped PhD and Postdoc members, which we discuss on The head of a Drosophila pupa. The developing down and handed the reins over to me. I’ve enjoyed page 5. Our PhD and Postdoc reps are working hard compound eye (green) is putting together this years’ newsletter. It’s been great to make the event bigger and better for next year! The composed of several hundred simple units called ommatidia to hear what our members have been up to, and I social events were well attended including the now arranged in an extremely hope you will enjoy reading it. infamous annual “Pub Quiz” and disco after the regular array. The giant conference dinner. Members will be relieved to know polyploidy cells of the fat body (red), the fly equivalent of the The 2016 BSCB/DB spring meeting, organised by our we aren’t including any photos from that here. mammalian liver and adipose committee members Buzz Baum (UCL), Silke tissue, occupy a big area of the Robatzek and Steve Royle, had a particular focus on In this issue, we highlight the great work the BSCB head. Cells and Tissue Architecture, Growth & Cell Division, has been doing to engage young scientists. -

EMBO Facts & Figures
excellence in life sciences Reykjavik Helsinki Oslo Stockholm Tallinn EMBO facts & figures & EMBO facts Copenhagen Dublin Amsterdam Berlin Warsaw London Brussels Prague Luxembourg Paris Vienna Bratislava Budapest Bern Ljubljana Zagreb Rome Madrid Ankara Lisbon Athens Jerusalem EMBO facts & figures HIGHLIGHTS CONTACT EMBO & EMBC EMBO Long-Term Fellowships Five Advanced Fellows are selected (page ). Long-Term and Short-Term Fellowships are awarded. The Fellows’ EMBO Young Investigators Meeting is held in Heidelberg in June . EMBO Installation Grants New EMBO Members & EMBO elects new members (page ), selects Young EMBO Women in Science Young Investigators Investigators (page ) and eight Installation Grantees Gerlind Wallon EMBO Scientific Publications (page ). Programme Manager Bernd Pulverer S Maria Leptin Deputy Director Head A EMBO Science Policy Issues report on quotas in academia to assure gender balance. R EMBO Director + + A Conducts workshops on emerging biotechnologies and on H T cognitive genomics. Gives invited talks at US National Academy E IC of Sciences, International Summit on Human Genome Editing, I H 5 D MAN 201 O N Washington, DC.; World Congress on Research Integrity, Rio de A M Janeiro; International Scienti c Advisory Board for the Centre for Eilish Craddock IT 2 015 Mammalian Synthetic Biology, Edinburgh. Personal Assistant to EMBO Fellowships EMBO Scientific Publications EMBO Gold Medal Sarah Teichmann and Ido Amit receive the EMBO Gold the EMBO Director David del Álamo Thomas Lemberger Medal (page ). + Programme Manager Deputy Head EMBO Global Activities India and Singapore sign agreements to become EMBC Associate + + Member States. EMBO Courses & Workshops More than , participants from countries attend 6th scienti c events (page ); participants attend EMBO Laboratory Management Courses (page ); rst online course EMBO Courses & Workshops recorded in collaboration with iBiology. -

Flow and Enzyme Biology
2010 ANNUAL MEETING THEMATIC MEETING SERIES BEGINS! July 2009 Stopped- Flow and Enzyme Biology American Society for Biochemistry and Molecular Biology contents JULY 2009 On the Cover: Britton Chance has provided innumerable contributions society news to the fields of biochemistry, 3 President’s Message biophysics, and biomedicine, including his design of 6 Washington Update the first stopped-flow 8 NIH News apparatuses (pictured). 32 special interest 18 The Department of Biological Chemistry at Johns Hopkins School of Medicine: 100 years at 100 Years of Excellence the Johns Hopkins 21 Honoring the Biochemist’s Biochemist: School of NIH Hosts the Stadtman Symposium Medicine. 18 2010 asbmb meeting 12 Lipids, Physiology, and Disease 14 Dealing with Insults: Genome Stability in the Face of Stress 16 Insights into the Biological Chemistry of RNA science focus 32 Britton Chance: Former Polymerase II: Olympian and Pioneer in Now Twice as Enzyme Kinetics and Faithful. Functional Spectroscopy 30 departments 2 Letters to the Editor 7 News from the Hill 10 Member Spotlight 22 Lipid News 23 Education and Training 26 Minority Affairs 28 Career Insights 30 BioBits podcast summary Listen to the latest JBC podcast featuring resources interviews with authors from the Thematic Scientific Meeting Calendar Minireview Series, “The biochemical basis for triplet repeat neurodegenerative diseases.” online only To hear this and other podcasts, go to www.asbmb.org/Interactive.aspx. July 2009 ASBMB Today 1 letters to the editor A monthly publication of The American Society for Biochemistry and Molecular Biology History Repeats Itself Officers Gregory A. Petsko President Heidi E. Hamm Past President in Big Pharma Mark A. -

Brigitte M. Jockusch Editor the Actin Cytoskeleton Handbook of Experimental Pharmacology
Handbook of Experimental Pharmacology 235 Brigitte M. Jockusch Editor The Actin Cytoskeleton Handbook of Experimental Pharmacology Volume 235 Editor-in-Chief James E. Barrett, Philadelphia Editorial Board V. Flockerzi, Homburg M.A. Frohman, Stony Brook, NY P. Geppetti, Florence F.B. Hofmann, Mu¨nchen M.C. Michel, Mainz C.P. Page, London W. Rosenthal, Berlin K. Wang, Beijing More information about this series at http://www.springer.com/series/164 Brigitte M. Jockusch Editor The Actin Cytoskeleton Editor Brigitte M. Jockusch Cell Biology, Life Sciences BRICS, TU Braunschweig Braunschweig, Germany ISSN 0171-2004 ISSN 1865-0325 (electronic) Handbook of Experimental Pharmacology ISBN 978-3-319-46369-8 ISBN 978-3-319-46371-1 (eBook) DOI 10.1007/978-3-319-46371-1 Library of Congress Control Number: 2016963070 # Springer International Publishing AG 2017 This work is subject to copyright. All rights are reserved by the Publisher, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfilms or in any other physical way, and transmission or information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed. The use of general descriptive names, registered names, trademarks, service marks, etc. in this publication does not imply, even in the absence of a specific statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use. The publisher, the authors and the editors are safe to assume that the advice and information in this book are believed to be true and accurate at the date of publication. -

Smutty Alchemy
University of Calgary PRISM: University of Calgary's Digital Repository Graduate Studies The Vault: Electronic Theses and Dissertations 2021-01-18 Smutty Alchemy Smith, Mallory E. Land Smith, M. E. L. (2021). Smutty Alchemy (Unpublished doctoral thesis). University of Calgary, Calgary, AB. http://hdl.handle.net/1880/113019 doctoral thesis University of Calgary graduate students retain copyright ownership and moral rights for their thesis. You may use this material in any way that is permitted by the Copyright Act or through licensing that has been assigned to the document. For uses that are not allowable under copyright legislation or licensing, you are required to seek permission. Downloaded from PRISM: https://prism.ucalgary.ca UNIVERSITY OF CALGARY Smutty Alchemy by Mallory E. Land Smith A THESIS SUBMITTED TO THE FACULTY OF GRADUATE STUDIES IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY GRADUATE PROGRAM IN ENGLISH CALGARY, ALBERTA JANUARY, 2021 © Mallory E. Land Smith 2021 MELS ii Abstract Sina Queyras, in the essay “Lyric Conceptualism: A Manifesto in Progress,” describes the Lyric Conceptualist as a poet capable of recognizing the effects of disparate movements and employing a variety of lyric, conceptual, and language poetry techniques to continue to innovate in poetry without dismissing the work of other schools of poetic thought. Queyras sees the lyric conceptualist as an artistic curator who collects, modifies, selects, synthesizes, and adapts, to create verse that is both conceptual and accessible, using relevant materials and techniques from the past and present. This dissertation responds to Queyras’s idea with a collection of original poems in the lyric conceptualist mode, supported by a critical exegesis of that work. -

Gazette 2018 7
GazetteWadham College 2018 2018 Gazette 2018 7 Contents Fellows' List 4 Features The Editor 8 The Warden 9 Wadham in 1618 67 The Domestic Bursar 12 Betjeman and Bowra 70 Staff List 14 The Remarkable Mrs Wadham (Senior) 73 The Finance Bursar 18 The 2nd Year 76 The Development Director 20 Book Reviews 78 The Senior Tutor 24 The Tutor for Access 26 College Record The Chapel and Choir 28 In Memoriam 86 The Sarah Lawrence Programme 30 Obituaries 88 The Library 32 Fellows' news 106 Emeritus Fellows' news 110 Clubs, Societies New Fellows 110 and Activities Visiting Fellows 113 1610 Society 36 Alumni news 115 Wadham Alumni Society 38 Degrees 118 Law Society 42 Donations 120 Medical Society 43 The Academic Record Wadham Alumni Golf Society 44 The Student Union 45 Graduate completions 140 MCR 46 Final Honour School results 143 Lennard Bequest Reading Party 48 First Public Examination results 145 Sports Prizes 147 Cricket 50 Scholarships and Exhibitions 149 Football 52 New Undergraduates 152 Rowing 54 New Graduates 156 Rugby 57 2019 Events 160 Netball 58 Squash 60 Tennis 60 Hockey 61 Water polo 62 Power lifting 62 www.wadham.ox.ac.uk Fellows’ list 5 Darren J. Dixon Thomas W. Simpson Samuel J. Williams Fellows’ list Professor of Organic Senior Research Fellow in Wadham College Law Chemistry, Knowles–Williams Philosophy and Public Policy Society Fellow by Special Fellow and Tutor in Organic and Senior Treasurer of Election Philip Candelas, FRS Martin G. Bureau Chemistry Amalgamated Clubs WARDEN Judy Z. Stephenson Rouse Ball Professor of Professor of Astrophysics Nathalie Seddon Susan M. -

2017 Edition 35 the Master
Clare News 2017 EDITION 35 THE MASTER In this issue Welcome from the Master Page 3 Achievements and Honours Page 5 Old Court Page 6 College News Page 10 Student Life Page 17 Sport Page 21 Page 22 Editor: Hannah Sharples Alumni Stories Design: www.cantellday.co.uk Photography: Hannah Sharples, Fellows’ Research Page 28 Helen Knowles Front Cover image: Nick Rutter Samuel Blythe Society Page 33 Contact: Publications Page 34 The Editor – Clare News, Clare College, Social Media Page 38 Trinity Lane, Cambridge CB2 1TL +44 (0)1223 333218 A Year in Clare Page 39 [email protected] www.clarealumni.com © Clare College 2017-18. All rights reserved. Forthcoming events are listed on the back cover 2 CLARE NEWS SUMMER 2014 THE MASTER Welcome from the Master I am delighted to present the latest edition of Clare News. As you will see from our contributors, a Clare education can take you far across the globe, and it is always a pleasure to keep our alumni and friends updated on what has been happening in College. Clare has had another excellent year, both Jane is a Senior Social Development Adviser academically and otherwise. One current at the UK Government’s Department for Clare Fellow and two alumnae of the International Development (DFID), and she College were this year elected to the Royal was awarded an OBE in the Queen’s 2015 Society, the self-governing Fellowship of Birthday Honours. During her time at DFID the most eminent scientists, engineers Jane’s work has included urban poverty, and technologists from the UK and the social exclusion, fragile states, and ending Commonwealth. -

EMBO Facts & Figures
excellence in life sciences young investigators|courses,workshops,conference series & symposia|installation grantees|long-term fellows|short-term fellows|policy, science & society|the EMBO Journal|EMBO reports|molecular systems biology|EMBO molecular medicine|global exchange|gold medal|the EMBO meeting|women in science| EMBO reports|molecular systems biology|EMBO molecular medicine|global exchange|gold medal|the EMBO meeting|women in science|young investigators|courses,workshops,conference series & symposia|installation grantees|long-term fellows|short-term fellows|policy, science & society|the EMBO Journal| global exchange|gold medal|the EMBO meeting|women in science|young investigators|long-term fellows|short-term fellows|policy, science & society|the EMBO Journal|courses,workshops,conference series & symposia|EMBO reports|molecular systems biology|EMBO molecular medicine|installation grantees| EMBO molecular medicine|installation grantees|long-term fellows|gold medal|molecular systems biology|short-term fellows|the EMBO meeting|womenReykjavik in science|young investigators|courses,workshops,conference series & symposia|global exchange|EMBO reports|policy, science & society|the EMBO Journal| gold medal|the EMBO meeting|women in science|young investigators|courses,workshops,conference series & symposia|global exchange|policy, science & society|the EMBO Journal|EMBO reports|molecular systems biology|EMBO molecular medicine|installation grantees|long-term fellows|short-term fellows| courses,workshops,conference series & symposia|global -

Open Space’ Meeting Called on 12 November 2015
What can Fellows do to support women in the biomedical workforce? A report of an ‘open space’ meeting called on 12 November 2015 1 Introduction On 12 November 2015 the Academy of Medical Sciences held a meeting for women Fellows to come together to discuss the question ‘What can Fellows do to support women in the biomedical workforce?’. Over 60 women Fellows registered to attend the event (see Annex 1 for names) The event was held using an open space format, which allows groups to self-organise and collaborate around a question of shared concern. The day began with an opening circle at which participants called sessions on the topics they wanted to discuss. This report contains reports of these sessions written by the Fellow who called them. Reports of all sessions were not received. It also includes a transcript of the closing session where participants summarised their thoughts about the day and ideas for next steps. Sessions called during the opening circle: • Women’s Attitudes • Sponsorship (vs Mentoring) • Lectures by women for women • How not to be patronising • The token woman • Fellows careers are not over • Recruitment to senior positions • Ambition limitation by social pressure • Networking - the pub? • Work / life balance • Being small • “Calm Down Dear!” - dealing with sexist comments • Deciding whether and when to have children • Invisibility • How do we get more women Fellows? • Changing the culture • Pink princess • Supporting transitions • Do we involve men? How? • Does language matter? • No more 1 in 4 • Science communication at a young age • Quotas? 30% • What when women numerically dominate? • What is special about women in science? • Style 2 Session reports Women’s attitudes 1. -

Identification and Characterization of a Set of Conserved and New Regulators of Cytoskeletal Organization, Cell Morphology and Migration
King’s Research Portal DOI: 10.1186/1741-7007-9-54 Document Version Publisher's PDF, also known as Version of record Link to publication record in King's Research Portal Citation for published version (APA): Bai, S. W., Herrera-Abreu, M. T., Rohn, J. L., Racine, V., Tajadura, V., Suryavanshi, N., Bechtel, S., Wiemann, S., Baum, B., & Ridley, A. J. (2011). Identification and characterization of a set of conserved and new regulators of cytoskeletal organization, cell morphology and migration. BMC Biology, 9, [54]. https://doi.org/10.1186/1741- 7007-9-54 Citing this paper Please note that where the full-text provided on King's Research Portal is the Author Accepted Manuscript or Post-Print version this may differ from the final Published version. If citing, it is advised that you check and use the publisher's definitive version for pagination, volume/issue, and date of publication details. And where the final published version is provided on the Research Portal, if citing you are again advised to check the publisher's website for any subsequent corrections. General rights Copyright and moral rights for the publications made accessible in the Research Portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognize and abide by the legal requirements associated with these rights. •Users may download and print one copy of any publication from the Research Portal for the purpose of private study or research. •You may not further distribute the material or use it for any profit-making activity or commercial gain •You may freely distribute the URL identifying the publication in the Research Portal Take down policy If you believe that this document breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. -

Lablinks: Tumour Microenvironment
LabLinks: Tumour Microenvironment Meeting Program 9:00–9:05 Opening Remarks 9:05–9:40 Anne Ridley, King’s College London Interactions of cancer cells with endothelial cells 9:40–10:15 Vincenzo Cerundolo, University of Oxford Tumour strategies to suppress cancer-specific immune responses 10:15–10:50 Karl Matter, University College London Signalling by epithelial junctions in LabLinks: Tumour cell migration and survival Microenvironment 10:50–11:15 Tea Break Monday, July 9, 2012 11:15–11:50 Fiona Watt, Centre for Stem Cells and 9:00am – 5:15pm GMT Regenerative Medicine, King’s College London B.5 Auditorium Defining dermal fibroblast lineages Francis-Wilkins Building King’s College London (Waterloo Campus) 11:50–12:25 Caetano Reis e Sousa, London Research London, UK Institute, Cancer Research UK Tumour cell death and immunity Organizers Debbie Taylor, Current Biology 12:25–1:30 Lunch (on your own) Rosy Hosking, Cell Anne Ridley, King’s College London 1:30–2:05 Kairbaan Hodivala-Dilke, Barts Cancer Institute, Caetano Reis e Sousa, London Research Institute, Queen Mary University of London Cancer Research UK The tumour microenvironment: Keynote Speaker Unlocking the journey Hans Clevers, Hubrecht University, Utrecht, The Netherlands 2:05–2:40 Erik Sahai, London Research Institute, Cancer Research UK To register go to: Cancer cell invasion in complex environments http://www.cell.com/cellpress/lablinks Registration is FREE 2:40–3:15 Fran Balkwill, Barts Cancer Institute, (Space is limited) Queen Mary University of London Tumour-promoting cytokine networks 3:10–3:40 Tea Break 3:40–4:15 Chris Marshall, Institute of Cancer Research Influence of microenvironment on modes of tumour cell migration 4:15–5:10 Keynote Speaker Hans Clevers, Hubrecht Institute, Utrecht Wnt signalling, Lgr5 stem cells and cancer www.cell.com 5:10–5:15 Closing Remarks.