Roundup Litigation: Using Discovery to Dissolve Doubt
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Agricultural Biotechnology: Benefits of Transgenic Soybeans
AGRICULTURAL BIOTECHNOLOGY: BENEFITS OF TRANSGENIC SOYBEANS Leonard P. Gianessi Janet E. Carpenter April 2000 National Center for Food and Agricultural Policy 1616 P Street, NW, First Floor Washington, DC 20036 Tel: 202-328-5048 Fax: 202-328-5133 [email protected] Preparation of this report was supported financially with a grant from the Rockefeller Foundation TABLE OF CONTENTS 1. Introduction 2. U.S. Soybean Production 3. Soybean Products 4. Soybean Physiology 5. Soybeans – Agronomic Factors 6. Soybean Genetic Improvements A. Introduction B. Reproductive Process C. Artificial Cross Breeding D. Mutation Breeding E. Transgenic Plants 7. Weed Competition – Soybeans 8. Weed Control in Soybeans: 1940’s – 1950’s 9. Herbicides – An Overview 10. Herbicide Use in Soybeans: 1960’s – 1995 A. Introduction B. Historical Overview 1. The Early 1960’s 2. Soil Applied Herbicides 3. Postemergence Herbicides 4. Sulfonylurea/Imidazolinone Herbicides 5. Burndown Herbicides C. Summary of Usage: 1995 11. Transgenic Herbicide Tolerant Soybeans A. Glyphosate – An Overview B. Performance of Roundup Ready Soybeans C. Herbicide Ratings D. Adoption Impacts: 1995 – 1998 1. Herbicide Costs 2. Soybean Yields 3. Returns 4. Other Aggregate Studies 5. Herbicide Treatments 6. Herbicide Use Amounts 7. Other Impacts 12. Summary and Conclusions 13. References Appendix 1: Soybean Processing – A Description 1. Introduction Soybeans and other crops have been improved genetically for many decades through traditional crop breeding – a technique that requires that species be sexually compatible. With the development of biotechnology methods, scientists have the ability to transfer single genes from one living organism into another, regardless of species or sexual compatibility. Varieties that are developed through the transfer of genes between species that are not sexually compatible are referred to as “transgenic.” Transgenic soybean plants have been developed with a gene from a soil bacteria that allows the use of an herbicide that would normally kill soybeans. -

Glyphosate and Cancer Risk: Frequently Asked Questions
May 2015 FACT SHEET GLYPHOSATE AND CANCER RISK: FREQUENTLY ASKED QUESTIONS WHY IS THERE CONCERN ABOUT showing higher rates of cancer in glyphosate-using GLYPHOSATE AND CANCER? The World farmers; and research showing that glyphosate damages Health Organization’s (WHO’s) cancer authorities – the DNA and chromosomes, one mechanism by which cancer International Agency for Research on Cancer (IARC) is induced.2 IARC’s full assessment is due out in 2016. – recently determined that glyphosate is “probably carcinogenic to humans” (Group 2A). Glyphosate is WHOSE ASSESSMENT IS MORE the most heavily used pesticide in the world thanks to RELIABLE: IARC OR EPA? IARC is the world’s widespread planting of Monsanto’s Roundup Ready crops, leading authority on cancer. Its glyphosate determination which are genetically engineered to survive spraying with was made by unanimous decision of 17 qualified scientists it. Use and exposure will increase still more if glyphosate- led by Dr. Aaron Blair, a distinguished epidemiologist resistant turfgrasses currently being developed for lawns, recently retired from the U.S. National Cancer Institute.3 playing fields and golf courses are introduced. IARC’s assessment is up-to-date, analyzing all the relevant available research, while EPA’s last comprehensive WHERE DO EPA AND WHO’S IARC STAND assessment of glyphosate occurred in 1993. IARC ON GLYPHOSATE’S CARCINOGENICITY? considered a broad range of evidence, including human In 1985, EPA classified glyphosate as a possible epidemiology and other peer-reviewed studies, while EPA carcinogen based on experiments showing tumors in did not assess epidemiology and relied almost entirely on glyphosate-treated rodents. -

Benefits and Safety of Glyphosate
TABLE OF CONTENTS 1 EXECUTIVE SUMMARY ………………………………………………………………….4 2 BENEFITS OF GLYPHOSATE …………………………………………………………...5 2.1 Benefits to agriculture in glyphosate-tolerant cropping systems …………………………..6 2.1.1 Expansion in agriculture and replacement of other herbicides ……………………. 6 2.1.2 Farm level benefits ………………………………………………………………………7 2.1.3 Impacts on conservation tillage ……………………………………………………….8 2.1.4 Value of U.S. commodity exports of glyphosate-tolerant crops …………………..10 2.2 Benefits to agriculture in non-glyphosate-tolerant crops ………………………………….11 2.2.1 Orchards and vineyards ………………………………………………………………11 2.2.2 Wheat …………………………………………………………………………………..12 2.2.3 Sugarcane ……………………………………………………………………………..12 2.2.4 Cover crops ……………………………………………………………………………13 2.3 Benefits outside of agriculture ……………………………………………………………….14 2.3.1 Highway, railroad and utility right of ways …………………………………………...14 2.3.2 Recreational settings ………………………………………………………………….15 2.3.3 Invasive and noxious weeds ………………………………………………………….16 2.3.4 Aquatic weeds …………………………………………………………………………18 2.4 Managing herbicide resistant weed biotypes ……………………………………………… 19 2.5 Potential impacts of losing access to glyphosate …………………………………………. 20 2.6 Policy considerations …………………………………………………………………………..21 3 SAFETY OF GLYPHOSATE ……………………………………………………………………...23 3.1 Glyphosate environmental fate and toxicology ……………………………………..24 3.2 Glyphosate ecotoxicology …………………………………………………………….24 3.3 Glyphosate and honey bees …………………………………………………………………..25 3.4 Glyphosate and soil biota ……………………………………………………………………..25 -

Can I Trust the World Health Organization on Glyphosate
MAIL Can I Trust the World Health Organization on Glyphosate (Roundup)? I’ve been advocating to eliminate glyphosate and other toxic pesticides to my local officials, and I’ve made some good progress. But right now, I need to push back against claims from some decision makers that IARC and the World Health Organization can’t be trusted when they say glyphosate is carcinogenic. Can you provide me with some additional back- ground about how the decision was made? And are there other countries or organizations that have agreed with their assessment? Carol, Rochester, NY © iStockphoto/Mihajlo Maricic Carol, The International Agency for Research on Cancer (IARC) is an findings were consistent with IARC’s conclusions on the agency within the United Nations’ World Health Organization. carcinogenicity of glyphosate. Importantly, cancer was not Since 1965, IARC has been the leading international body the only subject in ATSDR’s review of glyphosate. It also in making scientific determinations identifying carcinogenic reviewed the chemical’s effects on: body weight, pulmonary hazards to humans. IARC employs a “strength of evidence” and cardiovascular health, gastrointestinal and nervous assessment, basing the carcinogenicity of a chemical on systems, kidney and liver, skeletal system, endocrine system, whether it is capable of increasing the occurrence of malignant effects on the immune system, developmental and repro- growths, reducing their latency (time between exposure and ductive systems, and the eyes and skin. the onset of cancer), or increasing the severity or multiplicity Particular to glyphosate, the Danish government has con- of such growths. Prior to classifying a chemical, 17 experts curred with IARC’s cancer determination, Austria has moved from 11 countries analyze scientific studies and data for to ban the chemical, France is phasing the chemical out by approximately one year before meeting together in a Work- 2021, Germany is phasing use out by 2023, and the Nether- ing Group in an attempt to reach a consensus evaluation. -
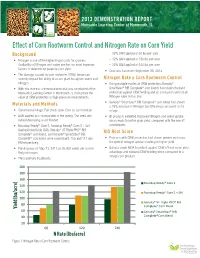
Effect of Corn Rootworm Control and Nitrogen Rate on Corn Yield
2013 Demonstration Report LEARNING the Monsanto Learning Center at Monmouth, IL MONSANTO CENTER ™ Effect of Corn Rootworm Control and Nitrogen Rate on Corn Yield Background – 32% UAN applied at 60 lbs per acre • Nitrogen is one of the highest input costs for growers. – 32% UAN applied at 120 lbs per acre Availability of Nitrogen and water are the two most important – 32% UAN applied at 240 lbs per acre factors in determining potential corn yield. • Corn was harvested September 25, 2013. • The damage caused by corn rootworm (CRW) larvae can severely impact the ability of a corn plant to capture water and Nitrogen Rate x Corn Rootworm Control nitrogen. • Using multiple modes of CRW protection (Genuity® • With this in mind, a demonstration trial was conducted at the SmartStax® RIB Complete® corn blend) had shown the best Monsanto Learning Center in Monmouth, IL to illustrate the protection against CRW feeding and an increase in yield at all value of CRW protection in high-pressure environments. Nitrogen rates in this trial. • Genuity® SmartStax® RIB Complete® corn blend had shown Materials and Methods a 75% increase in Nitrogen Use Efficiency compared to the • Conventional tillage: Fall chisel plow. Corn on corn rotation. refuge. • UAN applied and incorporated in the spring. The seed bed • Bt products exhibited improved Nitrogen and water uptake, established using a soil finisher which leads to better grain yield, compared with the non-Bt • Roundup Ready® Corn 2, Roundup Ready® Corn 2+ Soil counterparts. Applied Insecticide (SAI), Genuity® VT Triple PRO® RIB NIS Root Score Complete® corn blend, and Genuity® SmartStax® RIB Complete® corn blend were planted with 105 and 111 day • Products with CRW protection had shown greater root mass RM respectively. -

Monsanto's Roundup (Glyphosate)
Monsanto’s Roundup (Glyphosate) Exposed INDEPENDENT SCIENCE IDENTIFIES HEALTH AND ENVIRONMENTAL PROBLEMS EDITOR’s NOTE: This article summarizes recent research on glyphosate’s adverse effect on beneficial bac- teria essential to human health. For more information, see “Glyphosate Causes Cancer” in the Summer 2015 issue of Pesticides and You, “Agricultural Uses of Antibiotics Escalate Bacterial Resistance” in the Winter 2016–17 issue, and the Beyond Pesticides factsheet on glyphosate on the website at the Gateway on Pesticide Hazards and Safe Pest Management. An expanded and fully cited version of this article can be found on the Beyond Pesticides website. TERRY SHISTAR, Ph D lyphosate, which has been mistakenly GLYPHOSATE RISK ASSESSMENT characterized as a relatively innocuous EPA’s risk assessments rate glyphosate’s acute toxicity herbicide and is now known to pose as “relatively low.” In developmental toxicity studies multiple dangers to human health using pregnant rats and rabbits, glyphosate causes and the environment, demonstrates treatment-related effects in high dose groups, Gthe failure of the risk assessment paradigm for including diarrhea, decreased body weight gain, regulating toxic chemicals and the dangers of nasal discharge, and death. (EPA, 1993, 2006) EPA’s ignoring the importance of microbiota. controversial classification of glyphosate as a Group E carcinogen—evidence of non-carcinogenicity for Glyphosate (N-phosphono-methyl glycine) is a broad humans—is based on the lack of convincing evidence spectrum, post-emergent, non-selective, systemic of carcinogenicity in studies submitted to the agency herbicide used on non-cropland as well as a variety by Monsanto. However, contrary to EPA’s finding of crops. -

Roundup Ready® Soybeans? Yes
Post-Patent Roundup Ready ® Soybeans The last patent on the Roundup Ready® soybean trait expired a few years ago, and this flyer answers common questions that U.S. farmers ask about the use of that trait, patents, varieties, and saved seed. Is original Roundup Ready® soybean seed covered by other forms of intellectual property? While Monsanto’s* patents on the Roundup Ready® soybean trait have expired, most soybean seeds containing that trait are still covered by other forms of intellectual property in the United States, including patents on specific soybean varieties and Plant Variety Protection certificates that plant breeders obtain when they develop a new variety. Can a farmer legally save and plant original Roundup Ready® soybean seed? As of 2015, U.S. farmers may legally plant saved seed from some varieties of soybean containing the Roundup Ready® soybean trait. It is important that you check with your seed supplier to determine if a specific Roundup Ready® soybean variety is covered by other forms of intellectual property, and if so, the policy for saving seed of that variety. Will a farmer be able to purchase any soybean seed that contains the Roundup Ready® soybean trait? Ultimately, it is up to each seed company to determine if it will offer soybeans containing the Roundup Ready® soybean trait. Monsanto seed brands will not have any soybeans containing the Roundup Ready® soybean trait in their lineups. Monsanto developed the Roundup Ready 2 Yield® and Roundup Ready 2 Xtend® trait technologies to provide a higher yield opportunity for farmers, and many farmers have chosen to plant soybeans containing these newer technologies. -

The Seralini Affair: Degeneration of Science to Re‑Science? John Fagan1*, Terje Traavik2,3 and Thomas Bøhn2
Fagan et al. Environ Sci Eur (2015) 27:19 DOI 10.1186/s12302-015-0049-2 REVIEW Open Access The Seralini affair: degeneration of Science to Re‑Science? John Fagan1*, Terje Traavik2,3 and Thomas Bøhn2 Abstract A paper reporting findings relevant to safety of the genetically modified (GM) maize NK603 and the herbicide Roundup (Séralini et al., Food Chem Toxicol. 50:4221–4231, 2012) was retracted against the wishes of the authors, and subsequently republished in another peer-reviewed journal (Séralini et al. Environ Sci Europe, doi:10.1186/s12302- 014-0014-5, 2014). These events exemplify a trend in which disputes, between interest groups vying for retraction and republication of papers that report controversial results, overshadow the normal scientific process in which peer-reviewed publication stimulates new research, generating new empirical evidence that drives the evolution of scientific understanding. This paper reviews the current status of research on safety of NK603 maize and Roundup herbicide for human and livestock health, and attempts to glean from recent developments insights relevant to risk assessment policy for GM crops and pesticides, as well as relevant to the scientific process in general. Our analysis of currently published evidence confirms NK603 and Roundup are kidney and liver toxicants at levels below current regulatory thresholds. Consequently, the regulatory status of NK603, glyphosate and Roundup requires reevaluation. Additionally, preliminary evidence indicates Roundup and NK603, individually and in combination, may increase tumor incidence and mortality. Follow-up long-term carcinogenicity studies, using test animal strains and numbers of animals that assure robust conclusions, are required to confirm/refute this preliminary evidence. -

Glyphosate Glyphosate Herbicides (One Common Brand Name Is Roundup) Are the Mostly Commonly Used Herbicides in the U.S
HERBICIDE FACTSHEET Glyphosate Glyphosate herbicides (one common brand name is Roundup) are the mostly commonly used herbicides in the U.S. and the world. In agriculture they are widely used with genetically-modified glyphosate-tolerant crops, but they are also widely used in yards, gardens, and other nonagricultural areas. Symptoms of exposure to glyphosate include eye irritation, burning eyes, blurred vision, skin rashes, burning or itchy skin, nausea, sore throat, asthma and difficulty breathing, headache, lethargy, nose bleeds, and dizziness. Glyphosate and glyphosate-containing herbicides caused genetic damage in laboratory tests with human cells, as well as in tests with laboratory animals. Studies of farmers and other people exposed to glyphosate herbicides have shown that this exposure is linked with increased risks of the cancer non-Hodgkin’s lymphoma, miscarriages, and attention deficit disorder. For each of the hazards identified in these studies there are also laboratory studies with results that are consistent with the studies of exposed people. There is also laboratory evidence that glyphosate herbicides can reduce production of sex hormones. Studies of glyphosate contamination of water are limited, but new results indicate that it can commonly contaminate streams in both agricultural and urban areas. Problems with drift of glyphosate herbicides occur frequently. Only one other herbicide causes more drift incidents. Glyphosate herbicides caused genetic damage and damage to the immune system in fish. In frogs, glyphosate herbicides caused genetic damage and abnormal development. Application of glyphosate herbicides increases the severity of a variety of plant diseases. gardens. Home and garden use totals 5 BY CAROLINE COX Figure 1 over 5 million pounds per year. -

Review of GMO Safety Assessment Studies: Glyphosate Residues in Roundup Ready Crops Is an Ignored Issue Marek Cuhra1,2*
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Springer - Publisher Connector Cuhra Environ Sci Eur (2015) 27:20 DOI 10.1186/s12302-015-0052-7 REVIEW Open Access Review of GMO safety assessment studies: glyphosate residues in Roundup Ready crops is an ignored issue Marek Cuhra1,2* Abstract Background: Genetically modified glyphosate-tolerant cultivar varieties have been a commercial success widely known as Roundup Ready plants. As new glyphosate-tolerant varieties are introduced to satisfy agriculture demand, it is relevant to review the scientific evidence that documents the quality and safety of such biotechnology. Assess- ments of genetically modified glyphosate-tolerant plants are partly based on the reports from laboratory compari- sons with non-modified plants (near-isogenic relatives). Such comparative testing is typically performed as analysis of plant material composition and in animal feeding studies. The material for testing is typically produced in test-fields set up as model environments. Most of this research is planned, performed and reported by researchers employed by biotech industry companies. Perspective: The present paper aims to: (1) review 15 reports on compositional analyses of glyphosate-tolerant cultivars and 15 reports from animal feeding studies, (2) discuss recent data indicating glyphosate residue in Roundup Ready soybean, (3) outline recent developments of cultivars with increased tolerance to glyphosate. Findings: The reviewed industry studies show methodological flaws: glyphosate-tolerant GM crops are designed for use with glyphosate herbicide. However, glyphosate herbicides are often not applied in test-study cultivation. In the studies where glyphosate herbicides were applied to growing plants, the produced plant material was not ana- lyzed for glyphosate residues. -

United States District Court Eastern District of Missouri
Case: 4:20-cv-01145 Doc. #: 1 Filed: 08/26/20 Page: 1 of 38 PageID #: 1 UNITED STATES DISTRICT COURT EASTERN DISTRICT OF MISSOURI National Black Farmers Association JURY TRIAL DEMANDED Plaintiffs, Case No. v. COMPLAINT Monsanto Company Defendants. COMPLAINT COMES NOW Plaintiff National Black Farmers Association (“NBFA”), on behalf of its members, against Defendant Monsanto Company (“Monsanto”), alleging the following upon information and belief, except those allegations that pertain to NBFA and its members, which are based on personal knowledge: INTRODUCTION Monsanto’s Roundup® is a prevalent and yet highly dangerous product. Although it has long been marketed as an entirely safe herbicide whose main benefit is that it can be indiscriminately sprayed on fields planted with Monsanto’s “Roundup Ready” seeds, we now know that using Roundup® and its active ingredient, glyphosate, in this way causes cancer— including Non-Hodgkin’s Lymphoma (“NHL”), Hodgkin’s Lymphoma, Lymphoma, Multiple Myeloma and/or Leukemia. Bellwether plaintiffs have already litigated and prevailed on precisely such claims; other deserving plaintiffs are waiting to sue for their own damages; and certain class- action attorneys have already attempted to settle such claims, purporting to act on behalf of all other injured farmers. But conspicuously absent from these damages actions and purported settlements is any effort to get Roundup® off the shelf and protect farmers from the harm that it causes. The NBFA thus brings this action on behalf of its members, seeking an injunction that Case: 4:20-cv-01145 Doc. #: 1 Filed: 08/26/20 Page: 2 of 38 PageID #: 2 will either require Monsanto to stop selling its dangerous product, or else substantially change its behavior so that Black farmers are adequately warned about and protected from the potentially fatal results of using Roundup® in just the way that Monsanto has told (or, really, forced) them to do. -

Truflex-One-Pager-V7
READY WHEN YOU ARE You only get one shot. So go with your best option: TruFlex™ canola with Roundup Ready® technology. From seeding to harvest, you’ve only got 97 days* to maximize your yield potential. But you’re not worried. Because you’re growing TruFlex™ canola with Roundup Ready® Technology. Here’s how it can help you make the most of your season: IMPROVED CONTROL FLEXIBILITY IN SPRAY HIGHER YIELD POTENTIAL OF TOUGH WEEDS RATES AND TIMING THROUGH GENETICS AND TruFlex canola provides a wider application CROP SAFETY system allows for the control of window as compared to Roundup Ready® New genetics have packed a lot of yield harder-to-kill weeds like Canada potential into each TruFlex canola seed. thistle and wild buckwheat. delays your spray date, no problem – you New advances in trait technology help enable better weed control and crop safety compared to Roundup Ready canola. The TruFlex canola system also enables It’s a combination that could give you the opportunity to see much more yield herbicide application rate to help get the job potential at harvest time. done. *Spring B. napus usually matures 85 to 110 days after planting depending on hybrid and environmental conditions. Source: North Dakota State University Canola Production Field Guide (reviewed Jan. 2019). SYSTEM COMPARISON The ability to help effectively and consistently control both annual weeds and tough-to-control perennials – that’s what farmers are looking for, and it’s what the TruFlex canola with Roundup Ready Technology system enables. Canada Wild CONTROL DIFFICULT ANNUALS Thistle Buckwheat AND TOUGH PERENNIALS Control up to Roundup Ready® No control claim weed species Canada Thistle: Control with 44 fluid ounces per acre ® weed species1” in size with CRoundupanola Ready on labeled r27ates controlled Canola 27 controlled of Roundup PowerMAX herbicide up to the 6-leaf stage of canola development.