Adsorption of Colour from Pulp and Paper Mill Wastewaters Onto Diatomaceous Earths
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Memory Work on R ¯Ekohu (Chatham Islands) Kingsley Baird
Memory Connection Volume 3 Number 1 © 2019 The Memory Waka Hokopanopano Ka Toi Moriori (Reigniting Moriori Arts): Memory Work on R ¯ekohu (Chatham Islands) Kingsley Baird Hokopanopano Ka Toi Moriori (Reigniting Moriori Arts): Memory Work on R ¯ekohu (Chatham Islands)—Kingsley Baird Hokopanopano Ka Toi Moriori (Reigniting Moriori Arts): Memory Work on R ¯ekohu (Chatham Islands) Kingsley Baird Abstract Since European discovery of Re¯kohu (Chatham Islands) in 1791, the pacifist Moriori population declined rapidly as a result of introduced diseases (to which they had no immunity) and killing and enslavement by M¯aori iwi (tribes) from the New Zealand ‘mainland’ following their invasion in 1835. When (full-blooded) Tame Horomona Rehe—described on his headstone as the ‘last of the Morioris’— died in 1933, the Moriori were widely considered to be an extinct people. In February 2016, Moriori rangata m¯a tua (elders) and rangatehi (youth), artists and designers, archaeologists, a conservator and an arborist gathered at Ko¯ pinga Marae on Re¯kohu to participate in a w¯a nanga organized by the Hokotehi Moriori Trust. Its purpose was to enlist the combined expertise and commitment of the participants to hokopanopano ka toi Moriori (reignite Moriori arts)—principally those associated with r¯a kau momori (‘carving’ on living ko¯ pi trees)—through discussion, information exchange, speculation, toolmaking and finally, tree carving. In addition to providing a brief cultural and historical background, this paper recounts some of the memory work of the w¯a nanga from the perspective of one of the participants whose fascination for Moriori and the resilience of their culture developed from Michael King’s 1989 book, Moriori: A People Rediscovered. -

REFEREES the Following Are Amongst Those Who Have Acted As Referees During the Production of Volumes 1 to 25 of the New Zealand Journal of Forestry Science
105 REFEREES The following are amongst those who have acted as referees during the production of Volumes 1 to 25 of the New Zealand Journal of Forestry Science. Unfortunately, there are no records listing those who assisted with the first few volumes. Aber, J. (University of Wisconsin, Madison) AboEl-Nil, M. (King Feisal University, Saudi Arabia) Adams, J.A. (Lincoln University, Canterbury) Adams, M. (University of Melbourne, Victoria) Agren, G. (Swedish University of Agricultural Science, Uppsala) Aitken-Christie, J. (NZ FRI, Rotorua) Allbrook, R. (University of Waikato, Hamilton) Allen, J.D. (University of Canterbury, Christchurch) Allen, R. (NZ FRI, Christchurch) Allison, B.J. (Tokoroa) Allison, R.W. (NZ FRI, Rotorua) Alma, P.J. (NZ FRI, Rotorua) Amerson, H.V. (North Carolina State University, Raleigh) Anderson, J.A. (NZ FRI, Rotorua) Andrew, LA. (NZ FRI, Rotorua) Andrew, LA. (Telstra, Brisbane) Armitage, I. (NZ Forest Service) Attiwill, P.M. (University of Melbourne, Victoria) Bachelor, C.L. (NZ FRI, Christchurch) Bacon, G. (Queensland Dept of Forestry, Brisbane) Bagnall, R. (NZ Forest Service, Nelson) Bain, J. (NZ FRI, Rotorua) Baker, T.G. (University of Melbourne, Victoria) Ball, P.R. (Palmerston North) Ballard, R. (NZ FRI, Rotorua) Bannister, M.H. (NZ FRI, Rotorua) Baradat, Ph. (Bordeaux) Barr, C. (Ministry of Forestry, Rotorua) Bartram, D, (Ministry of Forestry, Kaikohe) Bassett, C. (Ngaio, Wellington) Bassett, C. (NZ FRI, Rotorua) Bathgate, J.L. (Ministry of Forestry, Rotorua) Bathgate, J.L. (NZ Forest Service, Wellington) Baxter, R. (Sittingbourne Research Centre, Kent) Beath, T. (ANM Ltd, Tumut) Beauregard, R. (NZ FRI, Rotorua) New Zealand Journal of Forestry Science 28(1): 105-119 (1998) 106 New Zealand Journal of Forestry Science 28(1) Beekhuis, J. -

LIST of MEMBERS on 1St MAY 1962
LIST OF MEMBERS ON 1st MAY 1962 HONORARY MEMBERS Champion, Sir Harry, CLE., D.Sc, M.A., Imperial Forestry Institute, Oxford University, Oxford, England Chapman, H. H., M.F., D.Sc, School of Forestry, Yale University, New Haven, Connecticutt, U.S.A, Cunningham, G. H., D.Sc, Ph.D., F.R.S.(N.S.), Plant Research Bureau, D.S.I.R., Auckland Deans, James, "Homebush", Darfield Entrican, A. R., C.B.E., A.M.I.C.E., 117 Main Road, Wellington, W.3 Foster, F. W., B.A. B.Sc.F., Onehuka Road, Lower Hutt Foweraker, C. E., M.A., F.L.S., 102B Hackthorne Road, Christchurch Jacobs, M. R., M.Sc, Dr.Ing., Ph.D., Dip.For., Australian Forestry School, Canberra, A.C.T. Larsen, C Syrach, M.Sc, Dr.Ag., Arboretum, Horsholm, Denmark Legat, C. E., C.B.E., B.Sc, Beechdene, Lower Bourne, Farnham, Surrey, England Miller, D., Ph.D., M.Sc, F.R.S., Cawthron Institute, Nelson Rodger, G. J., B.Sc, 38 Lymington Street, Tusmore, South Australia Spurr, S. TL, B.S., M.F., Ph.D., University of Michigan, Ann Arbor, Michigan, U.S.A. Taylor, N. IL, O.B.E., Soil Research Bureau, D.S.I.R., Wellington MEMBERS Allsop, F., N.Z.F.S., P.B., Wellington Armitage, M. F., N.Z.F.S., P.O. Box 513, Christchurch Barker, C. S., N.Z.F.S., P.B., Wellington Bay, Bendt, N.Z. Forest Products Ltd., Tokoroa Beveridge, A. E., Forest Reasearch Institute, P.B., Whakarewarewa, Rotorua Brown, C. H., c/o F.A.O., de los N.U., Casilla 10095, Santiago de Chile Buchanan, J. -

In Liquidation)
Liquidators’ First Report on the State of Affairs of Taratahi Agricultural Training Centre (Wairarapa) Trust Board (in Liquidation) 8 March 2019 Contents Introduction 2 Statement of Affairs 4 Creditors 5 Proposals for Conducting the Liquidation 6 Creditors' Meeting 7 Estimated Date of Completion of Liquidation 8 Appendix A – Statement of Affairs 9 Appendix B – Schedule of known creditors 10 Appendix C – Creditor Claim Form 38 Appendix D - DIRRI 40 Liquidators First Report Taratahi Agricultural Training Centre (Wairarapa) Trust Board (in Liquidation) 1 Introduction David Ian Ruscoe and Malcolm Russell Moore, of Grant Thornton New Zealand Limited (Grant Thornton), were appointed joint and several Interim Liquidators of the Taratahi Agricultural Training Centre (Wairarapa) Trust Board (in Liquidation) (the “Trust” or “Taratahi”) by the High Count in Wellington on 19 December 2018. Mr Ruscoe and Mr Moore were then appointed Liquidators of the Trust on 5th February 2019 at 10.50am by Order of the High Court. The Liquidators and Grant Thornton are independent of the Trust. The Liquidators’ Declaration of Independence, Relevant Relationships and Indemnities (“DIRRI”) is attached to this report as Appendix D. The Liquidators set out below our first report on the state of the affairs of the Companies as required by section 255(2)(c)(ii)(A) of the Companies Act 1993 (the “Act”). Restrictions This report has been prepared by us in accordance with and for the purpose of section 255 of the Act. It is prepared for the sole purpose of reporting on the state of affairs with respect to the Trust in liquidation and the conduct of the liquidation. -

Environmental Pest Plants
REFERENCES AND SELECTED BIBLIOGRAPHY © Crown Copyright 2010 145 Contract Report No. 2075 REFERENCES AND SELECTED BIBLIOGRAPHY Adams, J. 1885: On the botany of Te Aroha Mountain. Transactions and Proceedings of the New Zealand Institute 17: 275-281 Allaby, M. (ed) 1994: The Concise Oxford Dictionary of Ecology. Oxford University Press, Oxford, England. 415 pp. Allan, H. H. 1982: Flora of New Zealand. Vol 1. Government Printer, Wellington. Allen, D.J. 1983: Notes on the Kaimai-Mamaku Forest Park. New Zealand Forest Service, Tauranga (unpublished). 20 p. Allen R.B. and McLennan M.J. 1983, Indigenous forest survey manual: two inventory methods. Forest Research Institute Bulletin No. 48. 73 pp. Allen R.B. 1992: An inventory method for describing New Zealand vegetation. Forest Research Institute Bulletin No. 181. 25 pp. Anon 1975: Biological reserves and forest sanctuaries. What’s New in Forest Research 21. Forest Research Institute, Rotorua. 4 p. Anon 1982: Species list from Kopurererua Stream. New Zealand Wildlife Service National Habitat Register, May 1982. Bay of Plenty Habitat sheets, Folder 2, records room, Rotorua Conservancy. Anon 1983a: Reserve proposals. Northern Kaimai-Mamaku State Forest Park. Background notes for SFSRAC Meeting and Inspection, 1983. Tauranga. 12 pp. Anon 1983b: The inadequacy of the ecological reserves proposed for the Kaimai-Mamaku State Forest Park. Joint campaign on Native Forests, Nelson. 14 p. plus 3 references. Anon 1983c: Overwhelming support to save the Kaimai-Mamaku. Bush Telegraph 12: 1-2. Wellington. Anon 1989: Conservation values of natural areas on Tasman Forestry freehold and leasehold land. Unpublished report for Tasman Forestry Ltd, Department of Conservation and Royal Forest & Bird Protection Society. -

New Zealand 2021-2022 Guided Holidays
NEW ZEALAND 2021-2022 GUIDED HOLIDAYS 3-17 day Fully Curated Experiences 8-21 day Flexible Guided Holidays New Zealand Vista 14 DAYS AUCKLAND CHRISTCHURCH LAAC 14 INCLUDED EXPERIENCES HIGHLIGHTS Choose from a wide variety of sightseeing options – there is something to suit all tastes FLEXIBLE and travel styles. HOLIDAYS Explore famous Milford Sound and the spectacular Fiordland National Park, a UNESCO ICONIC World Heritage site. SITES Feel free to explore, with two nights in Rotorua and Queenstown to wine and TIME dine where you choose. FOR YOU Lunch is supplied through Eat My Lunch, an organisation that has given over 1.5 million SUSTAINABLE lunches to Kiwi kids. TRAVEL START END 1 Number of night stays Sightseeing stops AUCKLAND 1 Rotorua 2 Waitomo Caves Lake Taupo Huka Falls Mt. Ruapehu Franz Josef Glacier Picton 1 1 Wellington Mt. Cook Arthur’s Kaikoura Hokitika Pass TranzAlpine Train 1 Haast 1 1 CHRISTCHURCH Omarama Milford 1 Queenstown Sound 2 Oamaru 1 Te Anau 1 Dunedin TSS Earnslaw firstlighttravel.com WHY YOU'LL LOVE 8 Omarama – Dunedin Travel along the Waitaki River, which marks the THIS TRIP: boundary between the regions of Canterbury and Otago, and with its large catchment, it is an y It spans both of New Zealand’s ideal source of hydro-electric power. Continue incredible islands through the historic town of Oamaru en route to Dunedin, New Zealand’s southernmost y Waitomo’s Glowworms are an city, where your afternoon is at leisure. incredible natural phenomenon Consider an optional experience. Perhaps tour imposing Larnach Castle, take a wildlife y The scenic journey onboard the cruise out to the Otago Peninsula to spot TSS Earnslaw is unforgettable penguins and the Royal Albatross or experience Auckland an interactive tour of famous Speight’s y See cascading Milford Sound, Huka Brewery. -
Sector Guide from Napier/Hastings
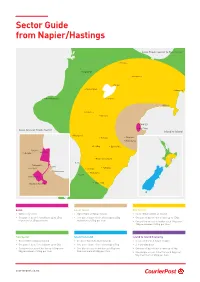
Sector Guide from Napier/Hastings Local Towns Sector to Two Sector • Rotorua Mangakino • Murupara • • Taupo • Taumarunui Gisborne • • New Plymouth • Turangi • Wairoa Ohakune • • Waiouru • NAPIER • HASTINGS Local & Local Towns Sector Island to Island • Whanganui • Taihape • Waipawa • Waipukurau • Feilding • Dannevirke Poraiti • Puketapu • NAPIER • • Palmerston North Foxton • Pakowhai • • Clive • Pahiatua • Fernhill • Shannon • Haumoana • Eketahuna • Levin HASTINGS • Havelock North • • Masterton Local Local Towns One Sector • Within city limits • Up to 75km within an Island • Up to 150km within an Island • One parcel base ticket allows up to 25kg • One parcel base ticket allows up to 25kg • One parcel base ticket allows up to 15kg maximum of 25kg per item maximum of 25kg per item • One yellow excess ticket for each 10kg over 15kg maximum of 25kg per item Two Sector Island to Island Island to Island Economy • Over 150km within an Island • Between North & South Islands • Between North & South Islands • One parcel base ticket allows up to 5kg • One parcel base ticket allows up to 5kg • 2-3 working days • One green excess ticket for each 5kg over • One blue excess ticket for each 5kg over • One parcel base ticket allows up to 5kg 5kg maximum of 25kg per item 5kg maximum of 25kg per item • One purple excess ticket for each 5kg over 5kg maximum of 25kg per item courierpost.co.nz CourierPost branch locations CourierPost Locations CourierPost Depot Address Auckland — City Auckland City CourierPost Depot 11 McDonald Street, Morningside, Auckland -

Border Report – Port of Tauranga and Rotorua Airport August 2013
Border Report – Port of Tauranga and Rotorua Airport August 2013 Purpose A preliminary report to understand the level of risk the Port of Tauranga (POT) and Rotorua Airport present to the Bay of Plenty kiwifruit industry with the intent of determining if the current level of protection is adequate. Background Biosecurity in New Zealand consists of a multi –layer system that begins offshore with pre-border activities, incorporates the border and continues post-border into New Zealand where it becomes a joint effort between central government, regional councils, industry, community groups, and all New Zealanders, (a paper describing this system in more detail can be found on the KVH website www.kvh.org.nz/kiwifruit_biosecurity_risks). This paper will review a single layer, border interventions at ports of entry. Any port of entry has the potential to bring unwanted pests and diseases into New Zealand that could be detrimental to the kiwifruit industry, however, given the high concentration of the kiwifruit industry in the Bay of Plenty, this report has focused on the ports of entry in the immediate proximity to this region, which are the Port of Tauranga and the Rotorua Airport. The Port of Tauranga is New Zealand’s second largest port by container volume, and a major stop on the cruise ship circuit. Rotorua Airport is an International Airport receiving two trans-Tasman flights a week. Imports into POT, cruise ships, and passenger traffic through Rotorua Airport are all potential pathways for risk items to enter New Zealand and each will be reviewed to provide an overview of operations, potential risks that each present and how these risks are being mitigated. -

Ecology, Management and History of the Forests of the Mamaku Plateau
Broekhuizen, P.; Nicholls, J.L.; Smale, M.C. 1985: A provisional list of vascular plant species: Rapurapu track, Kauri spur, and Rapurapu Gorge, Kaimai-Mamaku SF Park. Contributed by the Rotorua Botanical Society. Unpublished report held on file at Bay of Plenty Conservancy Office, Department of Conservation, Rotorua. [This work lists 135 indigenous species and 15 adventive species in the Rapurapu catchment, North Mamaku. It is arranged by lifeform within four vegetation types related to topography. Kauri (which is towards the lower southern extent of its range), six podocarp species and 47 fern species, which represents a strongly diverse fern flora for the relative size of the area surveyed, are recorded in the Rapurapu catchment, northern Mamaku. See Smale (1985) for botany of the catchment, and Bellingham et al. (1985) for botany of the general central and southern Mamaku Plateau—AEB.] Keywords: Rapurapu catchment, plant list, vegetation types, Rapurapu, kauri, Agathis australis, Kaimai Mamaku State Forest Park Brown, K.P.; Moller, H.; Innes, J.; Alterio, N. 1996: Calibration of tunnel tracking rates to estimate relative abundance of ship rats (Rattus rattus) and mice (Mus musculus) in a New Zealand forest. New Zealand Journal of Ecology 20: 271–275. [From the authors’ abstract:] Ship rat (Rattus rattus) and mouse (Mus musculus) density and habitat use were estimated by snap trapping and tracking tunnels at Kaharoa in central North Island, New Zealand. Eighty-one ship rats were caught in an effective trapping area of 12.4 ha. Extinction trapping gave an estimated density of 6.7 rats ha–1 (6.5–7.8 rats ha–1, 95% confidence intervals). -

Advanced Geothermal Geology
Advanced Geothermal Geology The objective of this course is to provide an advanced overview of geothermal geology and 10-14 FEB 2020 state-of-the-art methodology and simulation tools. Location: GNS Wairakei Research Centre & University of Auckland TOPICS COVERED This course complements JOGMEC’s introductory course and is useful for: • Geoscientists who have completed the JOGMEC course and want to advance their knowledge • Geoscientists working for oil/exploration companies interested in geothermal geology practices • Geothermal geoscientists interested in state-of-the-art / current practice methodology • Senior students and/or researchers in geothermal or complementary fi elds www.gns.cri.nz/geothermal-workshop Cost*: $6,560+GST/per person REGISTER ■ Limited number of spaces available (max 15 attendees) * Includes internal NZ travel, ■ Minimum number of attendees required (12) accommodation and food NOW ■ Registrations close 20 December 2019* * Excludes international travel ■ Course commences in Rotorua * GNS Science reserves the right to cancel the course if minimum numbers are not reached by 20 December 2019. A full refund will be arranged for all registration fees received. CONTACT Angus Howden [email protected] Advanced Geothermal Geology WORKSHOP PROGRAMME Day 1 Introduction to New Zealand Geothermal Development Meet with GNS geothermal specialists and local iwi (Māori) in Rotorua, to view the Rotorua 10 Feb 2020 Caldera and thermal manifestations (including Pohutu Geyser) at Te Puia thermal area, learn Rotorua – Taupō about the cultural importance of geothermal activity to New Zealand’s indigenous Māori and New Zealand’s approach to community engagement. Travel with GNS staff to Taupō, with stops at Ohaaki and Wairakei, where Contact Energy staff and GNS will describe the geology and structure of the Taupō Volcanic Zone, and history of geothermal development. -

Rekohu Report (2016 Newc).Vp
Rekohu REKOHU AReporton MorioriandNgatiMutungaClaims in the Chatham Islands Wa i 6 4 WaitangiTribunalReport2001 The cover design by Cliff Whiting invokes the signing of the Treaty of Waitangi and the consequent interwoven development of Maori and Pakeha history in New Zealand as it continuously unfoldsinapatternnotyetcompletelyknown AWaitangiTribunalreport isbn 978-1-86956-260-1 © Waitangi Tribunal 2001 Reprinted with corrections 2016 www.waitangi-tribunal.govt.nz Produced by the Waitangi Tribunal Published by Legislation Direct, Wellington, New Zealand Printed by Printlink, Lower Hutt, New Zealand Set in Adobe Minion and Cronos multiple master typefaces e nga mana,e nga reo,e nga karangaranga maha tae noa ki nga Minita o te Karauna. ko tenei te honore,hei tuku atu nga moemoea o ratou i kawea te kaupapa nei. huri noa ki a ratou kua wheturangitia ratou te hunga tautoko i kokiri,i mau ki te kaupapa,mai te timatanga,tae noa ki te puawaitanga o tenei ripoata. ahakoa kaore ano ki a kite ka tangi,ka mihi,ka poroporoakitia ki a ratou. ki era o nga totara o Te-Wao-nui-a-Tane,ki a Te Makarini,ki a Horomona ma ki a koutou kua huri ki tua o te arai haere,haere,haere haere i runga i te aroha,me nga roimata o matou kua mahue nei. e kore koutou e warewaretia. ma te Atua koutou e manaaki,e tiaki ka huri Contents Letter of Transmittal _____________________________________________________xiii 1. Summary 1.1 Background ________________________________________________________1 1.2 Historical Claims ____________________________________________________4 1.3 Contemporary Claims ________________________________________________9 1.4 Preliminary Claims __________________________________________________11 1.5 Rekohu, the Chatham Islands, or Wharekauri? _____________________________12 1.6 Concluding Remarks ________________________________________________13 2. -

QUARTERLY INFRASTRUCTURE REFERENCE GROUP UPDATE Q1: to 31 MARCH 2021 QUARTERLY IRG UPDATE 2 Q1: to 31 MARCH 2021
Building Together • Hanga Ngātahi QUARTERLY INFRASTRUCTURE REFERENCE GROUP UPDATE Q1: to 31 MARCH 2021 QUARTERLY IRG UPDATE 2 Q1: to 31 MARCH 2021 IRG PROGRAMME OVERVIEW .....................................3 PROGRESS TO DATE ................................................4 COMPLETED PROJECTS THIS QUARTER ...........................5 REGIONAL SUMMARY .............................................6 UPDATE BY REGION NORTHLAND ........................................................8 AUCKLAND .........................................................9 WAIKATO ...........................................................10 BAY OF PLENTY ....................................................11 GISBORNE ..........................................................12 HAWKE’S BAY ......................................................13 TARANAKI ..........................................................14 CONTENTS MANAWATŪ-WHANGANUI ........................................15 WELLINGTON .......................................................16 TOP OF THE SOUTH ................................................17 WEST COAST .......................................................18 CANTERBURY .......................................................19 OTAGO ..............................................................20 SOUTHLAND ........................................................21 NATIONWIDE .......................................................22 FULL PROJECT LIST ................................................23 GLOSSARY ..........................................................31