Pharmacology in MS Advanced Practice Management
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Comparison of Different Alpha-Blocker Combinations in Male Hypertensives with Refractory Lower Urinary Tract Symptoms
대한남성과학회지:제 29 권 제 3 호 2011년 12월 Korean J Androl. Vol. 29, No. 3, December 2011 http://dx.doi.org/10.5534/kja.2011.29.3.242 Comparison of Different Alpha-blocker Combinations in Male Hypertensives with Refractory Lower Urinary Tract Symptoms Keon Cheol Lee1, Jong Gu Kim2, Sung Yong Cho1, Joon Sung Jeon1, In Rae Cho1 Department of Urology, 1Inje University Ilsanpaik Hospital, Goyang, 2Happy Urology Clinic, Ansan, Korea =Abstract= Purpose: We compared the efficacy and safety profiles of dose increase, traditional combination methods, and combining different alpha blockers in hypertensive males with lower urinary tract symptom (LUTS) refractory to an initial dose of 4 mg doxazosin. Materials and Methods: Between 2000 and 2005, 374 male patients with LUTS and hypertension unresponsive to 4 weeks of 4 mg doxazosin were enrolled. The subjects were randomly classified into 3 groups, 8 mg/day of doxazosin (D group), 4 mg of doxazosin plus 0.2 mg/day of tamsulosin (DT group), and 4 mg doxazosin plus 5 mg/day finasteride (DF group). Patients were evaluated based on their International Prostate Symptom Score (IPSS), quality of life (QOL), uroflowmetry and blood pressure (BP) and adverse events (AEs) at the baseline and 3 and 12 months after treatment. Results: The 269 patients (71.9%) were followed for at least 1 year (D group n=84, DT group n=115, and DF group n=70). The clinical parameters before and after initial 4 mg/day doxazosin were not different among the 3 groups. IPSS improvement after 3 months and maximal flow rate (Qmax) improvement after 3 and 12 months were significantly higher in the D and DT groups than the DF group (p<0.05). -

Airway Receptors
Postgraduate Medical Journal (1989) 65, 532- 542 Postgrad Med J: first published as 10.1136/pgmj.65.766.532 on 1 August 1989. Downloaded from Mechanisms of Disease Airway receptors Peter J. Barnes Department of Thoracic Medicine, National Heart and Lung Institute, Dovehouse Street, London SW3 6L Y, UK. Introduction Airway smooth muscle tone is influenced by many indirect action which, in part, is due to activation of a hormones, neurotransmitters, drugs and mediators, cholinergic reflex, since the bronchoconstriction may which produce their effects by binding to specific be reduced by a cholinergic antagonist. Other surface receptors on airway smooth muscle cells. mediators may have a bronchoconstrictor effect Bronchoconstriction and bronchodilatation may which, in the case of adenosine, is due to mast cell therefore be viewed in terms of receptor activation or mediator release,2 or in the case of platelet-activating blockade and the contractile state of airway smooth factor due to platelet products.3 muscle is probably the resultant effect of interacting excitatory and inhibitory receptors. Epithelial-derived relaxantfactor It is important to recognize that airway calibre is not only the result of airway smooth muscle tone, but in Recently there has been considerable interest in the asthma it is likely that airway narrowing may also be possibility of a relaxant factor released from airway explained by oedema of the bronchial wall (resulting epithelial cells, which may be analogous to relaxant factor.4 The presence of from microvascular leakage) and to luminal plugging endothelial-derived by copyright. by viscous mucus secretions and extravasated plasma airway epithelium in bovine airways in vitro reduces proteins, which may be produced by a 'soup' of the sensitivity to and maximum contractile effect of mediators released from inflammatory cells, including spasmogens, such as histamine, acetylcholine or mast cells, macrophages and eosinophils. -

Provider- Pain Quick Reference Guide
A QUICK REFERENCE GUIDE (2019) PBM Academic Detailing Service Posttraumatic Stress Disorder A VA Clinician's Guide to Optimal Treatment of Posttraumatic Stress Disorder (PTSD) VA PBM Academic Detailing Service Real Provider Resources Real Patient Results Your Partner in Enhancing Veteran Health Outcomes VA PBM Academic Detailing Service Email Group [email protected] VA PBM Academic Detailing Service SharePoint Site https://vaww.portal2.va.gov/sites/ad VA PBM Academic Detailing Public Website http://www.pbm.va.gov/PBM/academicdetailingservicehome.asp Table of Contents Abbreviations . 1 PTSD Treatment Decision Aid . 3 VA/DoD 2017 Clinical Practice Guideline: Treatment of PTSD . 4 First-Line Treatment: Trauma-focused Psychotherapies with the Strongest Evidence . 5 First-Line Treatment: Trauma-Focused Psychotherapies with Sufficient Evidence . 6 Comparison of Antidepressants Studied in PTSD . 7 Recommended Antidepressant For PTSD: Dosing . 9 Additional Medications Studied in PTSD: Dosing . 11 Switching Antidepressants . 12 Antidepressants and Sexual Dysfunction . 14 i TOC (continued) Sexual Dysfunction Treatment Strategies . 16 Antidepressants and Hyponatremia . 17 Additional Pharmacotherapy Options for Veterans Refractory to Standard Treatments . 19 Discussing Benzodiazepine Withdrawal . 21 Prazosin Tips . 25 Prazosin Precautions . 26 Managing PTSD Nightmares in Veterans with LUTS Associated with BPH . 27 Other Medications Studied in PTSD-Related Nightmares . 29 References . 30 ii Abbreviations Anti-Ach = anticholinergic -

Oral Phentolamine: an Alpha-1, Alpha-2 Adrenergic Antagonist for the Treatment of Erectile Dysfunction
International Journal of Impotence Research (2000) 12, Suppl 1, S75±S80 ß 2000 Macmillan Publishers Ltd All rights reserved 0955-9930/00 $15.00 www.nature.com/ijir Oral phentolamine: an alpha-1, alpha-2 adrenergic antagonist for the treatment of erectile dysfunction I Goldstein1 1Department of Urology, Boston University School of Medicine, Boston, MA, USA Phentolamine mesylate is an alpha-1 and alpha-2 selective adrenergic receptor antagonist which has undergone clinical trials for erectile dysfunction treatment. Biochemical and physiological studies in human erectile tissue have revealed a high af®nity of phentolamine for alpha-1 and alpha-2 adrenergic receptors. Based on pharmacokinetic studies, it is suggested that 30±40 min following oral ingestion of 40 or 80 mg of phentolamine (Vasomax), the mean plasma phentolamine concentrations are suf®cient to occupy the alpha-1 and -2 adrenergic receptors in erectile tissue and thereby result in inhibition of adrenergic-mediated physiologic activity. In large multi-center, placebo-controlled pivotal phase III clinical trials, the mean change in the erectile function domain of the International Index of Erectile Function scores (Questions 1±5 and 15) from screening to the end of treatment was signi®cantly higher following use of active drug (40 mg and 80 mg) compared to placebo. Three to four times as many patients receiving phentolamine reported being satis®ed or very satis®ed compared with those receiving placebo. At doses of 40 mg and 80 mg respectively, 55% and 59% of men were able to achieve vaginal penetration with 51% and 53% achieving penetration on 75% of attempts. -

Therapeutic Drug Class
BUREAU FOR MEDICAL SERVICES WEST VIRGINIA MEDICAID EFFECTIVE PREFERRED DRUG LIST WITH PRIOR AUTHORIZATION CRITERIA 10/1/12 This is not an all-inclusive list of available covered drugs and includes only managed categories. Refer to cover page for complete list of rules governing this PDL. Version 2012.7b Prior authorization for a non-preferred agent in any category will be given only if there has been a trial of the preferred brand/generic equivalent or preferred formulation of the active ingredient, at a therapeutic dose, that resulted in a partial response with a documented intolerance. Prior authorization of a non-preferred isomer, pro-drug, or metabolite will be considered with a trial of a preferred parent drug of the same chemical entity, at a therapeutic dose, that resulted in a partial response with documented intolerance or a previous trial and therapy failure, at a therapeutic dose, with a preferred drug of a different chemical entity indicated to treat the submitted diagnosis. (The required trial may be overridden when documented evidence is provided that the use of these preferred agent(s) would be medically contraindicated.) Unless otherwise specified, the listing of a particular brand or generic name includes all legend forms of that drug. OTC drugs are not covered unless specified. PA criteria for non-preferred agents apply in addition to general Drug Utilization Review policy that is in effect for the entire pharmacy program, including, but not limited to, appropriate dosing, duplication of therapy, etc. The use of pharmaceutical samples will not be considered when evaluating the members’ medical condition or prior prescription history for drugs that require prior authorization. -

(ADAP) Formulary MAY 2021
Florida AIDS Drug Assistance Program (ADAP) Formulary MAY 2021 *Indicates controlled substance. Unless otherwise noted, all brands, strengths and dosage forms may be ordered pending wholesaler availability. Brand Name (listed for Generic Name Therapeutic Classification Pharmacologic Classification Antiretroviral drugs only) abacavir (ABC) Ziagen Antiretroviral NRTI abacavir/lamivudine (ABC/3TC) Epzicom Antiretroviral NRTI combo abacavir/lamivudine/zidovudine (ABC/3TC/AZT) Trizivir Antiretroviral NRTI combo atazanavir (ATV) Reyataz Antiretroviral PI atazanavir/cobicistat (ATV/COBI) Evotaz Antiretroviral PI/PK Enhancer bictegravir/emtricitabine/tenofovir alafenamide Biktarvy Antiretroviral INSTI/NRTI combo (BIC/FTC/TAF) cabotegravir (CAB) Vocabria Antiretroviral INSTI cabotegravir/rilpivirine (CAB/RPV) Cabenuva Antiretroviral INSTI/NNRTI combo cobicistat (COBI) Tybost Antiretroviral PK Enhancer darunavir (DRV) Prezista Antiretroviral PI darunavir/cobicistat (DRV/COBI) Prezcobix Antiretroviral PI/PK Enhancer darunavir/cobicistat/emtricitabine/tenofovir Symtuza Antiretroviral PI/NRTI combo alafenamide (DRV/COBI/FTC/TAF) dolutegravir (DTG) Tivicay Antiretroviral INSTI dolutegravir / lamivudine (DTG/3TC) Dovato Antiretroviral INSTI/NRTI combo dolutegravir/abacavir/lamivudine (DTG/ABC/3TC) Triumeq Antiretroviral INSTI/NRTI combo dolutegravir/rilpivirine (DTG/RPV) Juluca Antiretroviral INSTI/NNRTI combo doravirine (DOR) Pifeltro Antiretroviral NNRTI doravirine/lamivudine/tenofovir disoproxil fumarate Delstrigo Antiretroviral NNRTI/NRTI combo -

Alpha-Adrenoceptor Antagonists (Alpha-Blockers)
DRUG CLASSES ALPHA-ADRENOCEPTOR ANTAGONISTS (ALPHA-BLOCKERS) Alpha-blockers are usually used as add-on drugs in difficult to treat hypertension or where other drugs are poorly tolerated. Examples Doxazosin Indoramin Prazosin Terazosin Mechanism of action Commonly used alpha-blockers act selectively at post-ganglionic alpha1-receptors. Selective blockade of peripheral alpha1-receptors leads to vasodilatation and hence reduction in blood pressure. Pharmacokinetics Alpha-blockers are well absorbed after oral administration and undergo extensive hepatic metabolism. The main difference between agents is in elimination half-life: short with indoramin and prazosin and much longer with doxazosin and terazosin. Adverse effects First-dose postural hypotension associated with reflex cardioacceleration and palpitation. Much less common with doxazosin, especially in SR formulation. Vaso-vagal syncope after first dose if unable to mount a rapid heart rate response to hypotension. Caution particularly with terazosin. Headache Oedema Stress incontinence in women Drowsiness with Indoramin 1 Practical issues All alpha-blockers reduce blood pressure. However, doxazosin or terazosin are the preferred options because longer duration of action allows once daily administration. In sustained release formulation, the onset of action of doxazosin is gradual making first- dose hypotension less common. To avoid collapse due to hypotension, it is recomeneded to start terazosin with a low dose given at bedtime. Alpha-blockers are usually used with other agents in the treatment resistant hypertension. In addition to a role in hypertension, alpha-blockers are used to treat benign prostatic hypertrophy. Prazosin is also licensed for the treatment of Raynaud’s syndrome. Alpha- blockers can also be a useful choice in subjects suspected of having phaeochromocytoma. -

A Guide to the Administration of Medicines in the Perioperative
......„„.....NHS Grampian Guideline For The Administration Of Medicines In The Pen-Operative Period Co-ordinators: Consultation Group: Approver: Pre-Operative Assessment See Consultation list Medicine Guidelines and Pharmacist Policies Group Signature: Signature: ...i 0 0 a Identifier: Review Date: Date Approved: NHSG/Guid/Peri0p/ May 2022 May 2019 MGPG1026 Uncontrolled when printed Version 3 Executive Sign-Off This document has been endorsed by the Director of Pharmacy and Medicines Management Signature: Title: Guideline for the Administration of Medicines in the Peri- Operative Period Unique Identifier: NHSG/Guid/PeriOp/MGPG1026 Replaces: NHSG/Guid/PeriOp/MGPG697, Version 2 Across NHS Organisation Directorate Clinical Service Sub Boards Wide Department Area This controlled document shall not be copied in part or whole without the express permission of the author or the author’s representative. Lead Author/Co-ordinator: Pre-Operative Assessment Pharmacist Subject (as per document Clinical Guidance registration categories): Key word(s): Peri-operative medication guidance, gastro intestinal system, cardiovascular system, respiratory, central nervous, infections, endocrine, obstetrics, gynaecology, urinary tract, malignant, nutrition, blood, musculoskeletal, joint, corticosteroid treatment, alternative routes, antiepileptic Process Document: Policy, Guideline Protocol, Procedure or Guideline Document application: NHS Grampian Purpose/description: This policy is designed to be used by pharmacy, nursing and medical staff at pre-assessment -

Supplemental Table 2 Revised
Supplemental Table 2: Additional data validated by the expert panel for inclusion in PIM-Check PIM-Check additional data: Potentially Inappropriate Medications for Patients in Internal Medicine checklist This supplemental table includes PIM-check statements’ additional data. It includes statements’ rational and references and when appropriate recommendations, remarks, and useful links. This tool was validated using a 2-round Delphi method including 40 international experts from Belgium, France, Quebec and Switzerland. Their median level of agreement and useful rating during the second round are indicated for each statement. This is not a replacement for a clinical and biological evaluation by a clinician. The proposals are only applicable in the event there is no patient-specific contraindication. Some drugs listed may not be available in each country. A glossary of all abbreviations used in this tool is available at the end of the table. The *, **, *** symbols refer to information contained in table 3. CARDIOLOGY HEART FAILURE Rational Reduced risk of hospitalisation for heart failure and reduced risk of premature death. Recommendations Suggested dosing regimens: Titration up to effective doses or up to the maximum tolerated dose, while monitoring renal function. Maximum suggested dosing regimens: ACEI: captopril 50 mg TID, enalapril 10–20 mg BID, fosinopril 40 mg QD, lisinopril 20–40 mg QD, perindopril 8–16 mg QD, quinapril 20 mg BID, ramipril 5 mg BID, trandolapril 4 1 mg QD UP ARB: candesartan 32 mg QD, valsartan 160 mg BID, losartan -

Comparison of Alpha Blockers in Treatment of Premature Ejaculation: a Pilot Clinical Trial
Iranian Red Crescent Medical Journal. 2013 October; 15(10): e13805. DOI: 10.5812.ircmj.13805 Research Article Published Online 2013 October 05. Comparison of Alpha Blockers in Treatment of Premature Ejaculation: A Pilot Clinical Trial 1, * 2 3 1 1 Yigit Akin , Hakan Gulmez , Mutlu Ates , Aliseydi Bozkurt , Baris Nuhoglu 1 Harran University School of Medicine, Department of Urology, Sanliurfa, Turkey 2 Department of Family Medicine, Baskent University School of Medicine, Ankara, Turkey 3 Department of Urology, Afyon Kocatepe University School of Medicine, Afyonkarahisar, Turkey *Corresponding Author : Yigit Akin, Harran University School of Medicine, Department of Urology, 63100, Sanliurfa, Turkey. Tel: +90-5065334999, Fax: +90-4462161819, E-mail: [email protected] Received: ; Revised: ; Accepted: July 25, 2013 August 23, 2013 September 05, 2013 Background: Premature ejaculation (PE) is the most common sexual disorder in men and studies reported prevalence up to 30% (1, 2). PE is not a life-threatening medical condition but it influences the quality of life (QoL). Objectives: The aim of this study was to compare the efficiency, and safety of alpha blocker drugs in the treatment of patients with premature ejaculation (PE). Additionally we investigated the quality of life (QoL) in patients with PE who were treated with alpha blocker drugs. Materials and Methods: This study was a pilot clinical trial. Prospectively documented 108 patients with PE were treated and were followed-up in urology outpatient clinic. All patients were divided into 5 groups according to used alpha blocker agents which were determined by simple randomization. Silodosin 4mg (Group 1, n = 21), tamsulosin hydrochloride 0.4mg (Group 2, n = 23), alfuzosin 10mg (Group 3, n = 22), terazosin 5mg (Group 4, n = 21), doksazosin mesylate 4mg (Group5, n = 21), were used for treatment. -
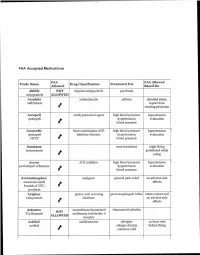
Faa-Meds.Pdf
FAA Accepted Medications. FAA FAA Allow Trade Name Drug Classificationti Treatment For Allowed Based On ed Abilify NOT atypical antipsychotic psychosis aripiprazole ALLOWED Accolate antiasthmatic asthma detailed status zafirlukast report from t treating physician Accupril antihypertensive agent high blood pressure hypertension quinapril hypertension evaluation blood pressure Accuretic fixed combination ACE- high blood pressure hypertension ' quinapril inhibitor/diuretic hypertension evaluation HCTZ blood pressure Accutane acne treatment night flying isotretonoin prohibited while le using Aceon ACE inhibitor high blood pressure hypertension ' perindopril erbumine hypertension evaluation t blood pressure Acetaminophen analgesic general pain relief no adverse side numerous label effects brands of OTC products Aciphex gastric acid secreting gastroesophageal reflux status report and rabeprazole inhibitor no adverse side effects Actemra recombinant humanized rheumatoid arthritis NOT Tocilizumab antihuman interleukin 6 ALLOWED receptor Actifed antihistamine allergies 12 hour wait actifed allergic rhinitis before flying common cold Actigall bile acid reduction agent gall stones status report and ursidiol no adverse side effects liver function studies Actonel bone resorption inhibitor osteoporosis status report and risedronate sodium no adverse side effects Actoplus insulin sensitizing agent Diabetes, Type 2 diabetes pioglitazone evaluation metformin Actos oral diabetes agent Diabetes, Type 2 diabetes pioglitazone evaluation Adalat calcium channel -

Graph Analysis of Β2 Adrenergic Receptor Structures: a “Social Network” of GPCR Residues Samuel Sheftel1, Kathryn E Muratore1, Michael Black2 and Stefano Costanzi1,3*
Sheftel et al. In Silico Pharmacology 2013, 1:16 http://www.in-silico-pharmacology.com/content/1/1/16 ORIGINAL RESEARCH Open Access Graph analysis of β2 adrenergic receptor structures: a “social network” of GPCR residues Samuel Sheftel1, Kathryn E Muratore1, Michael Black2 and Stefano Costanzi1,3* Abstract Purpose: G protein-coupled receptors (GPCRs) are a superfamily of membrane proteins of vast pharmaceutical interest. Here, we describe a graph theory-based analysis of the structure of the β2 adrenergic receptor (β2 AR), a prototypical GPCR. In particular, we illustrate the network of direct and indirect interactions that link each amino acid residue to any other residue of the receptor. Methods: Networks of interconnected amino acid residues in proteins are analogous to social networks of interconnected people. Hence, they can be studied through the same analysis tools typically employed to analyze social networks – or networks in general – to reveal patterns of connectivity, influential members, and dynamicity. We focused on the analysis of closeness-centrality, which is a measure of the overall connectivity distance of the member of a network to all other members. Results: The residues endowed with the highest closeness-centrality are located in the middle of the seven transmembrane domains (TMs). In particular, they are mostly located in the middle of TM2, TM3, TM6 or TM7, while fewer of them are located in the middle of TM1, TM4 or TM5. At the cytosolic end of TM6, the centrality detected for the active structure is markedly lower than that detected for the corresponding residues in the inactive structures. Moreover, several residues acquire centrality when the structures are analyzed in the presence of ligands.