Zestril® (Lisinopril) Tablets Label
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Comparison of the Tolerability of the Direct Renin Inhibitor Aliskiren and Lisinopril in Patients with Severe Hypertension
Journal of Human Hypertension (2007) 21, 780–787 & 2007 Nature Publishing Group All rights reserved 0950-9240/07 $30.00 www.nature.com/jhh ORIGINAL ARTICLE A comparison of the tolerability of the direct renin inhibitor aliskiren and lisinopril in patients with severe hypertension RH Strasser1, JG Puig2, C Farsang3, M Croket4,JLi5 and H van Ingen4 1Technical University Dresden, Heart Center, University Hospital, Dresden, Germany; 2Department of Internal Medicine, La Paz Hospital, Madrid, Spain; 31st Department of Internal Medicine, Semmelweis University, Budapest, Hungary; 4Novartis Pharma AG, Basel, Switzerland and 5Novartis Institutes for Biomedical Research, Cambridge, MA, USA Patients with severe hypertension (4180/110 mm Hg) LIS 3.4%). The most frequently reported AEs in both require large blood pressure (BP) reductions to reach groups were headache, nasopharyngitis and dizziness. recommended treatment goals (o140/90 mm Hg) and At end point, ALI showed similar mean reductions from usually require combination therapy to do so. This baseline to LIS in msDBP (ALI À18.5 mm Hg vs LIS 8-week, multicenter, randomized, double-blind, parallel- À20.1 mm Hg; mean treatment difference 1.7 mm Hg group study compared the tolerability and antihyperten- (95% confidence interval (CI) À1.0, 4.4)) and mean sitting sive efficacy of the novel direct renin inhibitor aliskiren systolic blood pressure (ALI À20.0 mm Hg vs LIS with the angiotensin converting enzyme inhibitor À22.3 mm Hg; mean treatment difference 2.8 mm Hg lisinopril in patients with severe hypertension (mean (95% CI À1.7, 7.4)). Responder rates (msDBPo90 mm Hg sitting diastolic blood pressure (msDBP)X105 mm Hg and/or reduction from baselineX10 mm Hg) were 81.5% and o120 mm Hg). -

Ace Inhibitors (Angiotensin-Converting Enzyme)
Medication Instructions Ace Inhibitors (Angiotensin-Converting Enzyme) Generic Brand Benazepril Lotensin Captopril Capoten Enalapril Vasotec Fosinopril Monopril Lisinopril Prinivil, Zestril Do not Moexipril Univasc Quinapril Accupril stop taking Ramipril Altace this medicine Trandolapril Mavik About this Medicine unless told ACE inhibitors are used to treat both high blood pressure (hypertension) and heart failure (HF). They block an enzyme that causes blood vessels to constrict. This to do so allows the blood vessels to relax and dilate. Untreated, high blood pressure can damage to your heart, kidneys and may lead to stroke or heart failure. In HF, using by your an ACE inhibitor can: • Protect your heart from further injury doctor. • Improve your health • Reduce your symptoms • Can prevent heart failure. Generic forms of ACE Inhibitors (benazepril, captopril, enalapril, fosinopril, and lisinopril) may be purchased at a lower price. There are no “generics” for Accupril, Altace Mavik, and of Univasc. Thus their prices are higher. Ask your doctor if one of the generic ACE Inhibitors would work for you. How to Take Use this drug as directed by your doctor. It is best to take these drugs, especially captopril, on an empty stomach one hour before or two hours after meals (unless otherwise instructed by your doctor). Side Effects Along with needed effects, a drug may cause some unwanted effects. Many people will not have any side effects. Most of these side effects are mild and short-lived. Check with your doctor if any of the following side effects occur: • Fever and chills • Hoarseness • Swelling of face, mouth, hands or feet or any trouble in swallowing or breathing • Dizziness or lightheadedness (often a problem with the first dose) Report these side effects if they persist: • Cough – dry or continuing • Loss of taste, diarrhea, nausea, headache or unusual fatigue • Fast or irregular heartbeat, dizziness, lightheadedness • Skin rash Special Guidelines • Sodium in the diet may cause you to retain fluid and increase your blood pressure. -

Use of Antipsychotic Medications in Nursing Facility Residents
POLICY Use of Antipsychotic Medications in Nursing Facility Residents Preamble The Office of Inspector General of the U. S. Department of Health and Human Services issued a report in May 2011 finding that 14% of elderly nursing home residents had Medicare claims for atypical antipsychotic drugs.1 In addition, 83% of the above Medicare claims for atypical antipsychotics for these elderly nursing home residents were for off---label indications. In 2005, the U. S. Food and Drug Administration required manufacturers of atypical antipsychotic medications to include a boxed warning that these antipsychotics may increase the risk of death in elderly persons with psychosis related to dementia. This warning was expanded to all antipsychotic drugs in 2008. The OIG report found that 88% of the use of atypical antipsychotics was in persons for whom this warning is applicable. As part of the report, OIG conducted medical record reviews on 600 elderly nursing facility residents. They found that 22% of the atypical antipsychotic medications were not administered in accordance with standards from the Centers for Medicare & Medicaid Services relating to unnecessary drug use in nursing homes. This controversial report from the OIG has generated concern about the use of antipsychotics in patients with dementia, and also criticism of the report itself.2 ---4 This document will provide additional background on this subject and a position statement from the American Society of Consultant Pharmacists. Background Understanding the findings and implications of the OIG report on use of atypical antipsychotics in nursing home residents requires placing the report findings into a proper context. Shown below are key points to understanding this report. -

Therapeutic Class Overview Fluoroquinolones
Therapeutic Class Overview Fluoroquinolones INTRODUCTION The fluoroquinolones are broad-spectrum antibiotics grouped into generations based on their spectrum of activity (Bolon 2011). ○ First generation agents, which are structurally quinolones rather than fluoroquinolones, possess activity against aerobic gram-negative bacteria but are not effective against aerobic gram-positive bacteria or anaerobes. The first generation agents (eg, nalidixic acid, cinoxacin) are no longer on the market. ○ Second generation agents, the original fluoroquinolones, contain a fluorine atom at position C-6. These agents offer improved coverage against gram-negative bacteria and moderately improved gram-positive coverage. The available second generation fluoroquinolones include ciprofloxacin, levofloxacin, and ofloxacin. Lomefloxacin and norfloxacin are second generation agents which are no longer on the market. ○ Third generation agents achieve greater potency against gram-positive bacteria, particularly pneumococci, and also possess good activity against anaerobes. All 3 of the third generation agents, gatifloxacin, grepafloxacin, and sparfloxacin, were removed from the market due to toxicities. ○ Fourth generation fluoroquinolones have superior coverage against pneumococci and anaerobes. The available agent is moxifloxacin. Trovafloxacin, was removed from the market due to toxicities, and there is a drug shortage of gemifloxacin. ○ The most recently approved fluoroquinolone, delafloxacin, has an even broader spectrum of antibiotic activity and is commonly referred to as a “next generation” fluoroquinolone. The fluoroquinolones have been used to treat a variety of infections including urinary tract infections, sinusitis, lower respiratory tract infections, intra-abdominal infections, infectious diarrhea, skin and skin structure infections, sexually transmitted diseases, and bacterial prostatitis. A few of the agents also have Food and Drug Administration (FDA) approval for inhalational anthrax and plague. -

Angioedema After Long-Term Use of an Angiotensin-Converting Enzyme Inhibitor
J Am Board Fam Pract: first published as 10.3122/jabfm.10.5.370 on 1 September 1997. Downloaded from BRIEF REPORTS Angioedema After Long-Term Use of an Angiotensin-Converting Enzyme Inhibitor Adriana]. Pavietic, MD Angioedema is an uncommon adverse effect of cyclobenzaprine, her angioedema was initially be angiotensin-converting enzyme (ACE) inhib lieved to be an allergic reaction to this drug, and itors. Its frequency ranges from 0.1 percent in pa it was discontinued. She was also given 125 mg of tients on captopril, lisinopril, and quinapril to 0.5 methylprednisolone intramuscularly. Her an percent in patients on benazepril. l Most cases are gioedema did not improve, and she returned to mild and occur within the first week of treat the clinic the following day. She was seen by an ment. 2-4 Recent reports indicate that late-onset other physician, who discontinued lisinopril, and angioedema might be more prevalent than ini her symptoms resolved within 24 hours. Mter her tially thought, and fatal cases have been de ACE inhibitor was discontinued, she experienced scribed.5-11 Many physicians are not familiar with more symptoms and an increased frequency of late-onset angioedema associated with ACE in palpitations, but she had no change in exercise hibitors, and a delayed diagnosis can have poten tolerance. A cardiologist was consulted, who pre tially serious consequences.5,8-II scribed the angiotensin II receptor antagonist losartan (initially at dosages of 25 mg/d and then Case Report 50 mg/d). The patient was advised to report any A 57-year-old African-American woman with symptoms or signs of angioedema immediately copyright. -
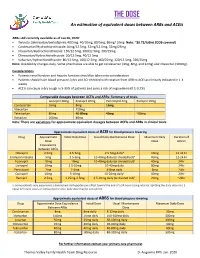
THE DOSE an Estimation of Equivalent Doses Between Arbs and Aceis
THE DOSE An estimation of equivalent doses between ARBs and ACEIs ARBs still currently available as of Jan 26, 2020: Twynsta (telmisartan/amlodipine): 40/5mg. 40/10mg, 80/5mg, 80mg/ 10mg Note: ~$0.73/tablet (ODB covered) Candesartan/Hydrochlorothiazide:16mg/12.5mg, 32mg/12.5mg, 32mg/25mg Irbesartan/Hydrochlorothiazide: 150/12.5mg, 300/12.5mg, 300/25mg Olmesartan/Hydrochlorothiaizde: 20/12.5mg, 40/12.5mg Valsartan/Hydrochlorothiazide: 80/12.5mg, 160/12.5mg, 160/25mg, 320/12.5mg, 320/25mg Note: Availability changes daily. Some pharmacies are able to get candesartan (4mg, 8mg, and 32mg) and irbesartan (300mg). Considerations Patients renal function and hepatic function should be taken into consideration Patients should have blood pressure, lytes and SCr checked with rotation from ARB to ACEI as clinically indicated in 1-4 weeks ACEIs can cause a dry cough in 5-35% of patients and carry a risk of angioedema (0.1-0.2%) Comparable dosages between ACEIs and ARBs- Summary of trials Lisinopril 20mg Enalapril 20mg Perindopril 4mg Ramipril 10mg Candesartan 16mg 8mg 16mg Irbesartan 150mg Telmisartan 80mg 40-80mg 40mg ~80mg Valsartan 160mg 80mg Note: There are variations for approximate equivalent dosages between ACEIs and ARBs in clinical trials. Approximate equivalent doses of ACEI for blood pressure lowering Drug Approximate Initial Daily Dose Usual Daily Maintenance Dose Maximum Daily Duration of Dose Dose Action Equivalence Between ACEIs Cilazapril 2.5mg 2.5-5mg 2.5-5mg dailya 10mg 12-24 hr Enalapril maleate 5mg 2.5-5mg 10-40mg daily (or divided bid)a 40mg 12-24 hr Fosinopril 10mg 10mg 10-40mg daily (or divided bid)a 40mg 24hr Lisinopril 10mg 2.5-10mg 10-40mg daily 80mg 24hr Perindopril 2mg 2-4mg 4-8mg daily 8mg 24hr Quinapril 10mg 5-10mg 10-20mg dailya 40mg 24hr Ramipril 2.5mg 1.25mg-2.5mg 2.5-10mg daily (or divided bid)a 20mg ~24hr a: Some patients may experience a diminished antihypertensive effect toward the end of a 24-hour dosing interval. -

AVELOX Or Other Quinolones (4, 5.6) AVELOX® Safely and Effectively
HIGHLIGHTS OF PRESCRIBING INFORMATION -------------------------------CONTRAINDICATIONS----------------------------- These highlights do not include all the information needed to use Known hypersensitivity to AVELOX or other quinolones (4, 5.6) AVELOX® safely and effectively. See full prescribing information for AVELOX. -----------------------WARNINGS AND PRECAUTIONS----------------------- AVELOX® (moxifloxacin hydrochloride) Tablet, film-coated • Increased risk of tendinitis and tendon rupture. This risk is further increased AVELOX® (moxifloxacin hydrochloride) Injection, solution for IV use in older patients usually over 60 years of age, in patients taking Initial U.S. Approval: 1999 corticosteroids, and in patients with kidney, heart or lung transplants. To reduce the development of drug-resistant bacteria and maintain the Discontinue if pain or inflammation in a tendon occurs. (5.1, 8.5) effectiveness of AVELOX and other antibacterial drugs, AVELOX should be • Prolongation of the QT interval and isolated cases of torsade de pointes has used only to treat or prevent infections that are proven or strongly suspected to been reported. Avoid use in patients with known prolongation, hypokalemia, be caused by susceptible bacteria. and with drugs that prolong the QT interval. (5.2, 7.4, 8.5). Use caution in patients with proarrhythmic conditions such as clinically significant bradycardia or acute myocardial ischemia. (5.2) WARNING: • Serious and sometimes fatal hypersensitivity reactions, including Fluoroquinolones, including AVELOX®, are associated with an anaphylactic reactions, may occur after first or subsequent doses. increased risk of tendinitis and tendon rupture in all ages. This risk is Discontinue drug use at first sign of skin rash, jaundice or any other sign of further increased in older patients usually over 60 years of age, in hypersensitivity. -

Depo-Provera Contraceptive Injection Should Not Be Used As a Long
HIGHLIGHTS OF PRESCRIBING INFORMATION • Known hypersensitivity to Depo-Provera CI (medroxyprogesterone These highlights do not include all the information needed to use DEPO- acetate or any of its other ingredients). (4) PROVERA CI safely and effectively. See full prescribing information for • Significant liver disease. (4) DEPO-PROVERA CI. • Undiagnosed vaginal bleeding. (4) DEPO-PROVERA CI (medroxyprogesterone acetate) injectable ------------------------------WARNINGS AND PRECAUTIONS----------------- suspension, for intramuscular use Thromboembolic Disorders: Discontinue Depo-Provera CI in patients who develop thrombosis (5.2) Initial U.S. Approval: 1959 Cancer Risks: Monitor women with breast nodules or a strong family WARNING: LOSS OF BONE MINERAL DENSITY history of breast cancer carefully. (5.3) • Women who use Depo-Provera Contraceptive Injection (Depo- Ectopic Pregnancy: Consider ectopic pregnancy if a woman using Provera CI) may lose significant bone mineral density. Bone loss is Depo-Provera CI becomes pregnant or complains of severe abdominal greater with increasing duration of use and may not be completely pain. (5.4) reversible. (5.1) Anaphylaxis and Anaphylactoid Reactions: Provide emergency medical • It is unknown if use of Depo-Provera Contraceptive Injection during treatment. (5.5) adolescence or early adulthood, a critical period of bone accretion, Liver Function: Discontinue Depo-Provera CI if jaundice or will reduce peak bone mass and increase the risk for osteoporotic disturbances of liver function develop (5.6) Carbohydrate Metabolism: Monitor diabetic patients carefully. (5.11) fracture in later life. (5.1) • Depo-Provera Contraceptive Injection should not be used as a long- ----------------------------------ADVERSE REACTIONS--------------------------- term birth control method (i.e., longer than 2 years) unless other Most common adverse reactions (incidence >5%) are: menstrual irregularities birth control methods are considered inadequate. -

Black Box Warnings 2
First Quarter 2014 P&T Update Vol. XI, Issue 1 Formulary Addition/Deletion Special Points of 1. Argatroban - Argatroban is a selective and short-acting direct thrombin inhibitor FDA Interest: approved for the prophylaxis or treatment of thrombosis or venous thromboembolism in patients with heparin-induced thrombocytopenia (HIT), and of coronary artery • P&T Update-Formulary Addition/Deletion thrombosis during percutaneous coronary intervention in patients at risk of HIT. The formulary addition request was submitted by the Pharmacy Department with • Policy and Procedures/ endorsement from Cardiology and Hematology representatives. Floorstock Update Formulary addition – approved • Black Box Warnings 2. Argatroban dosing nomogram – approved • New Guidelines Released by 3. Dinoprostone 0.5mg gel (Prepidil®) – The Obstetrics, Gynecology, and Women’s Health Joint National Committee Department no longer use dinoprostone gel for cervical ripening. The request to (JNC) 8 for High Blood remove dinoprostone from the formulary was approved. Pressure Management in Formulary deletion – approved. Adults 4. Hydroxocobalamin (Cyanokit®) - Hydroxocobalamin is indicated for treating cyanide toxicity. The UH ED department recommended adding hydroxocobalamin to the formulary in place of Cyanide antidote kit. The committee voted to approve the formulary addition of hydroxocobalamin. A mini-FMEA on hydroxocobalamin was also submitted. Formulary addition – approved 5. Cyanide antidote kit (Sodium Nitrate + Amyl Nitrate + Sodium Thiosulfate) - Cyanide antidote kit has been manufacturer discontinued. The committee voted to delete this product from the formulary. Formulary deletion – approved 6. Pertuzumab (Perjeta®) – Pertuzumab is a monoclonal antibody approved by the FDA to use in combination with trastuzumab and docetaxel as first-line therapy for HER2- positive metastatic breast cancer. -

Angiotensin-Converting Enzyme (ACE) Inhibitors
Angiotensin-Converting Enzyme (ACE) Inhibitors Summary Blood pressure reduction is similar for the ACE inhibitors class, with no clinically meaningful differences between agents. Side effects are infrequent with ACE inhibitors, and are usually mild in severity; the most commonly occurring include cough and hypotension. Captopril and lisinopril do not require hepatic conversion to active metabolites and may be preferred in patients with severe hepatic impairment. Captopril differs from other oral ACE inhibitors in its rapid onset and shorter duration of action, which requires it to be given 2-3 times per day; enalaprilat, an injectable ACE inhibitor also has a rapid onset and shorter duration of action. Pharmacology Angiotensin Converting Enzyme Inhibitors (ACE inhibitors) block the conversion of angiotensin I to angiotensin II through competitive inhibition of the angiotensin converting enzyme. Angiotensin is formed via the renin-angiotensin-aldosterone system (RAAS), an enzymatic cascade that leads to the proteolytic cleavage of angiotensin I by ACEs to angiotensin II. RAAS impacts cardiovascular, renal and adrenal functions via the regulation of systemic blood pressure and electrolyte and fluid balance. Reduction in plasma levels of angiotensin II, a potent vasoconstrictor and negative feedback mediator for renin activity, by ACE inhibitors leads to increased plasma renin activity and decreased blood pressure, vasopressin secretion, sympathetic activation and cell growth. Decreases in plasma angiotensin II levels also results in a reduction in aldosterone secretion, with a subsequent decrease in sodium and water retention.[51035][51036][50907][51037][24005] ACE is found in both the plasma and tissue, but the concentration appears to be greater in tissue (primarily vascular endothelial cells, but also present in other organs including the heart). -

Hyponatremia Induced by Angiotensin Converting Enzyme Inhibitors and Angiotensin
DOI: 10.7860/JCDR/2018/31983.11754 Original Article Hyponatremia Induced by Angiotensin Converting Enzyme Inhibitors and Angiotensin Pharmacology Section Receptor Blockers-A Pilot Study S BHUVANESHWARI1, PRAKASH VEL SANKHYA SAROJ2, D VIJAYA3, M SABARI SOWMYA4, R SENTHIL KUMAR5 ABSTRACT Results: Among all, 48% (24 out of 50) of the study population Introduction: Hyponatremia, serum sodium <135 mmol/L, can administered with ACEI and ARB developed hyponatremia. result in neurological manifestation in acute cases, may lead Predisposition to develop hyponatremia was high in males to seizures and coma. Angiotensin Converting Enzyme Inhibitor compared to females. Incidence of hyponatremia was 62.5% (10 (ACEI) and Angiotensin II Receptor Blockers (ARB) are drugs out of 16) in the age group of 56-75 years. Though, incidence of that have been commonly prescribed for the treatment of hyponatremia was 54.5% (18 out of 33) in ACEI group compared hypertension and cardiac diseases. It has become important to 35.2% (6 out of 17) in ARB group, but it was not statistically to evaluate and investigate the incidence of hyponatremia on significant. The study also revealed that metosartan had a higher consumption of these drugs. association with hyponatremia compared to other drugs. Aim: To determine the susceptibility of patients on ACEI and Conclusion: Hyponatremia was induced in nearly 50% of ARB to hyponatremia and to ascertain the drug producing patients taking ACEI and ARB. The incidence of hyponatremia notable hyponatremia among ACEI and ARB. among patients on these two drugs did not show statistical variation. Metosartan showed a higher incidence of Materials and Methods: The study was conducted in a tertiary hyponatremia compared to enalapril, ramipril, captopril, care hospital. -

Lisinopril (Ethics) Lisinopril Tablets 5 Mg, 10 Mg, 20 Mg
New Zealand Data Sheet Lisinopril (Ethics) Lisinopril Tablets 5 mg, 10 mg, 20 mg 1. NAME OF THE MEDICINAL PRODUCT LISINOPRIL (Ethics) 5 mg, tablet LISINOPRIL (Ethics) 10 mg, tablet LISINOPRIL (Ethics) 20 mg, tablet 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Lisinopril (Ethics) tablets come in three strengths and contain lisinopril dihydrate equivalent to 5 mg, 10 mg or 20 mg lisinopril. 3. PHARMACEUTICAL FORM 5 mg tablet: A light pink coloured, circular, biconvex uncoated tablet. The tablet has a break line and is embossed with ‘5’ on one side and embossed with ‘BL’ on the other side. 10 mg tablet: A light pink coloured, circular, biconvex uncoated tablet. The tablet is embossed with ‘10’ on one side and embossed with ‘BL’ on the other side. 20 mg tablet: A pink, circular, biconvex uncoated tablet. The tablet is embossed with ‘20’ on one side and embossed with ‘BL’ on the other side. Lisinopril dihydrate. The chemical name for lisinopril dihydrate is N-[N-[(1S)-1-carboxy-3- phenylpropyl]-L-lysyl]-L-proline dihydrate. Its structural formula is: C21H31N3O5.2H2O, Molecular weight: 441.53, CAS No.: 83915-83-7 Lisinopril dihydrate is a white to off-white crystalline powder that is soluble in water, sparingly soluble in methanol and practically insoluble in ethanol. A synthetic peptide derivative, lisinopril dihydrate is an oral long acting angiotensin converting enzyme inhibitor. It is a lysine analogue of enalaprilat (active metabolite of enalapril). 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Lisinopril is indicated in the treatment of essential hypertension and in renovascular hypertension. It may be used alone or concomitantly with other classes of antihypertensive agents.