Abstracts of Phd Theses
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Characterization of Bacterial Communities Associated
www.nature.com/scientificreports OPEN Characterization of bacterial communities associated with blood‑fed and starved tropical bed bugs, Cimex hemipterus (F.) (Hemiptera): a high throughput metabarcoding analysis Li Lim & Abdul Hafz Ab Majid* With the development of new metagenomic techniques, the microbial community structure of common bed bugs, Cimex lectularius, is well‑studied, while information regarding the constituents of the bacterial communities associated with tropical bed bugs, Cimex hemipterus, is lacking. In this study, the bacteria communities in the blood‑fed and starved tropical bed bugs were analysed and characterized by amplifying the v3‑v4 hypervariable region of the 16S rRNA gene region, followed by MiSeq Illumina sequencing. Across all samples, Proteobacteria made up more than 99% of the microbial community. An alpha‑proteobacterium Wolbachia and gamma‑proteobacterium, including Dickeya chrysanthemi and Pseudomonas, were the dominant OTUs at the genus level. Although the dominant OTUs of bacterial communities of blood‑fed and starved bed bugs were the same, bacterial genera present in lower numbers were varied. The bacteria load in starved bed bugs was also higher than blood‑fed bed bugs. Cimex hemipterus Fabricus (Hemiptera), also known as tropical bed bugs, is an obligate blood-feeding insect throughout their entire developmental cycle, has made a recent resurgence probably due to increased worldwide travel, climate change, and resistance to insecticides1–3. Distribution of tropical bed bugs is inclined to tropical regions, and infestation usually occurs in human dwellings such as dormitories and hotels 1,2. Bed bugs are a nuisance pest to humans as people that are bitten by this insect may experience allergic reactions, iron defciency, and secondary bacterial infection from bite sores4,5. -

The Porcine Nasal Microbiota with Particular Attention to Livestock-Associated Methicillin-Resistant Staphylococcus Aureus in Germany—A Culturomic Approach
microorganisms Article The Porcine Nasal Microbiota with Particular Attention to Livestock-Associated Methicillin-Resistant Staphylococcus aureus in Germany—A Culturomic Approach Andreas Schlattmann 1, Knut von Lützau 1, Ursula Kaspar 1,2 and Karsten Becker 1,3,* 1 Institute of Medical Microbiology, University Hospital Münster, 48149 Münster, Germany; [email protected] (A.S.); [email protected] (K.v.L.); [email protected] (U.K.) 2 Landeszentrum Gesundheit Nordrhein-Westfalen, Fachgruppe Infektiologie und Hygiene, 44801 Bochum, Germany 3 Friedrich Loeffler-Institute of Medical Microbiology, University Medicine Greifswald, 17475 Greifswald, Germany * Correspondence: [email protected]; Tel.: +49-3834-86-5560 Received: 17 March 2020; Accepted: 2 April 2020; Published: 4 April 2020 Abstract: Livestock-associated methicillin-resistant Staphylococcus aureus (LA-MRSA) remains a serious public health threat. Porcine nasal cavities are predominant habitats of LA-MRSA. Hence, components of their microbiota might be of interest as putative antagonistically acting competitors. Here, an extensive culturomics approach has been applied including 27 healthy pigs from seven different farms; five were treated with antibiotics prior to sampling. Overall, 314 different species with standing in nomenclature and 51 isolates representing novel bacterial taxa were detected. Staphylococcus aureus was isolated from pigs on all seven farms sampled, comprising ten different spa types with t899 (n = 15, 29.4%) and t337 (n = 10, 19.6%) being most frequently isolated. Twenty-six MRSA (mostly t899) were detected on five out of the seven farms. Positive correlations between MRSA colonization and age and colonization with Streptococcus hyovaginalis, and a negative correlation between colonization with MRSA and Citrobacter spp. -
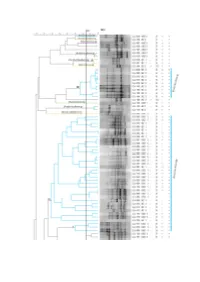
Supplementary File 1 (PDF, 1566 Kib)
Figure S1. Dendrogram based on cluster analysis of fingerprinting PCR profiles of the isolates from A. longifolia nodules, using the Pearson correlation coefficient and the unweighted pair-group method with arithmetic mean algorithm (UPGMA). 84% was the cut-off level below which isolates could be considered different. On the right are represented: isolate identification (CJJ xxx), zone from where it was isolated (UBZ/BZ x), Gram test result, morphology (rods (B) or cocci (CC)), catalase test result and oxidase test result, both (+) or (-). Colours are according to the phylum/class into each genus belong to: Proteobacteria/α-proteobacteria (blue), Proteobacteria/β-proteobacteria (orange), Proteobacteria/γ-proteobacteria (green), firmicutes/Bacilli (purple) and actinobacteria/Actinobacteria (yellow). Roman numbering identifies clusters. Isolates are identified up to genus level. Table S1. Identification of bacterial isolates obtained from unburnt and burnt zones by BLAST analysis of the 16S rRNA gene sequences. GenBank accession numbers are also indicated. GenBank % Pairwise Isolate ID Identification accession Closest hit by BLAST analysis1 Identity numbers CJJ 003 Bradyrhizobium sp. MT465339 Bradyrhizobium sp. MH612954 100% CJJ 008 Bradyrhizobium sp. MT465340 Bradyrhizobium sp. Z94815 100% CJJ 010 Bradyrhizobium sp. MT465341 Bradyrhizobium cytisi MK370569 98.1% CJJ 013 Bradyrhizobium pachyrhizi MT465342 Bradyrhizobium pachyrhizi KP769443 100% CJJ 017 Bradyrhizobium sp. MT465343 Bradyrhizobium cytisi MK370569 99.3% CJJ 020 Bradyrhizobium sp. MT465344 Bradyrhizobium sp. DQ202229 99.5% CJJ 024 Bradyrhizobium cytisi MT465345 Bradyrhizobium cytisi MK30569 100% CJJ 025 Paraburkholderia phytofirmans MT465346 Paraburkholderia phytofirmans NR_102845 100% Bradyrhizobium ganzhouense NR_133706 99.8% CJJ 026 Bradyrhizobium sp. MT465347 Bradyrhizobium rifense NR_116361 99.8% CJJ 027 Althererythrobacter sp. MT465348 Althererythrobacter sp. -

Active Microorganisms Thrive Among Extremely Diverse Communities in Cloud Water Pierre Amato, Muriel Joly, Ludovic Besaury, Anne Oudart, Najwa Taïb, Anne I
Active microorganisms thrive among extremely diverse communities in cloud water Pierre Amato, Muriel Joly, Ludovic Besaury, Anne Oudart, Najwa Taïb, Anne I. Moné, Laurent Deguillaume, Anne-Marie Delort, Didier Debroas To cite this version: Pierre Amato, Muriel Joly, Ludovic Besaury, Anne Oudart, Najwa Taïb, et al.. Active microorganisms thrive among extremely diverse communities in cloud water. PLoS ONE, Public Library of Science, 2017, 12 (8), 10.1371/journal.pone.0182869. hal-01598571 HAL Id: hal-01598571 https://hal.archives-ouvertes.fr/hal-01598571 Submitted on 6 Nov 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. RESEARCH ARTICLE Active microorganisms thrive among extremely diverse communities in cloud water Pierre Amato1*, Muriel Joly1, Ludovic Besaury1, Anne Oudart1,2, Najwa Taib2, Anne I. MoneÂ2, Laurent Deguillaume3, Anne-Marie Delort1, Didier Debroas2 1 Universite Clermont Auvergne, CNRS, Institut de Chimie de Clermont-Ferrand, Clermont-Ferrand, France, 2 Universite Clermont Auvergne, CNRS, Laboratoire Microorganismes: GeÂnome et Environnement, Clermont-Ferrand, France, 3 Universite Clermont Auvergne, CNRS, Observatoire de Physique du Globe, Clermont-Ferrand, France a1111111111 a1111111111 * [email protected] a1111111111 a1111111111 a1111111111 Abstract Clouds are key components in Earth's functioning. -

Meta-Analysis Cum Machine Learning Approaches Address the Structure
www.nature.com/scientificreports OPEN Meta‑analysis cum machine learning approaches address the structure and biogeochemical potential of marine copepod associated bacteriobiomes Balamurugan Sadaiappan1,5, Chinnamani PrasannaKumar1,5, V. Uthara Nambiar1, Mahendran Subramanian2,3,4 & Manguesh U. Gauns1* Copepods are the dominant members of the zooplankton community and the most abundant form of life. It is imperative to obtain insights into the copepod‑associated bacteriobiomes (CAB) in order to identify specifc bacterial taxa associated within a copepod, and to understand how they vary between diferent copepods. Analysing the potential genes within the CAB may reveal their intrinsic role in biogeochemical cycles. For this, machine‑learning models and PICRUSt2 analysis were deployed to analyse 16S rDNA gene sequences (approximately 16 million reads) of CAB belonging to fve diferent copepod genera viz., Acartia spp., Calanus spp., Centropages sp., Pleuromamma spp., and Temora spp.. Overall, we predict 50 sub‑OTUs (s‑OTUs) (gradient boosting classifers) to be important in fve copepod genera. Among these, 15 s‑OTUs were predicted to be important in Calanus spp. and 20 s‑OTUs as important in Pleuromamma spp.. Four bacterial s‑OTUs Acinetobacter johnsonii, Phaeobacter, Vibrio shilonii and Piscirickettsiaceae were identifed as important s‑OTUs in Calanus spp., and the s‑OTUs Marinobacter, Alteromonas, Desulfovibrio, Limnobacter, Sphingomonas, Methyloversatilis, Enhydrobacter and Coriobacteriaceae were predicted as important s‑OTUs in Pleuromamma spp., for the frst time. Our meta‑analysis revealed that the CAB of Pleuromamma spp. had a high proportion of potential genes responsible for methanogenesis and nitrogen fxation, whereas the CAB of Temora spp. had a high proportion of potential genes involved in assimilatory sulphate reduction, and cyanocobalamin synthesis. -

Contrasting Patterns of Microbial Communities in Glacier Cryoconite of Nepali Himalaya and Greenland, Arctic
sustainability Article Contrasting Patterns of Microbial Communities in Glacier Cryoconite of Nepali Himalaya and Greenland, Arctic Purnima Singh 1,2, Masaharu Tsuji 3 , Shiv Mohan Singh 4,5,* and Nozomu Takeuchi 6 1 Parvatibai Chowgule College of Arts & Science, Goa 403602, India; [email protected] 2 NPDF-Science and Engineering Research Board, New Delhi 110 070, India 3 Department of Materials Chemistry, Asahikawa College, National Institute of Technology, Hokkaido 071-8142, Japan; [email protected] 4 Polar Biology Laboratory, National Centre for Antarctic and Ocean Research, Goa 403804, India 5 Department of Botany, Institute of Science, Banaras Hindu University, Varanasi 221005, India 6 Department of Earth Sciences, Graduate School of Science, Chiba University, Chiba 263-8522, Japan; [email protected] * Correspondence: [email protected] Received: 10 June 2020; Accepted: 30 July 2020; Published: 11 August 2020 Abstract: To understand the microbial composition and diversity patterns, cryoconite granules were collected from two geographical areas, i.e., Nepali Himalaya and Greenland, Arctic. 16S rRNA, ITS and the D1/D2 domain sequencing techniques were used for characterization of microbial communities of the four glaciers. The total 13 species of bacteria such as Bacillus aryabhattai, Bacillus simplex, Brevundimonas vesicularis, Cryobacterium luteum, Cryobacterium psychrotolerans, Dermacoccus nishinomiyaensis, Glaciihabitans tibetensis, Leifsonia kafniensis, Paracoccus limosus, Polaromonas glacialis, -

Biodegradation of Microcystins by Bacterial Consortia
UC Irvine UC Irvine Electronic Theses and Dissertations Title Biodegradation of Microcystins by Bacterial Consortia Permalink https://escholarship.org/uc/item/8c01g1jj Author Cheung, Yuen Ming Publication Date 2015 License https://creativecommons.org/licenses/by/4.0/ 4.0 Peer reviewed|Thesis/dissertation eScholarship.org Powered by the California Digital Library University of California UNIVERSITY OF CALIFORNIA, IRVINE Biodegradation of Microcystins by Bacterial Consortia THESIS submitted in partial satisfaction of the requirements for the degree of MASTER OF SCIENCE in Engineering with a specialization in Environmental Engineering by Yuen Ming Cheung Thesis Committee: Professor Sunny Jiang, Ph.D, Chair Professor Betty Olson, Ph.D Professor Diego Rosso, Ph.D 2015 © 2015 Yuen Ming Cheung TABLE OF CONTENTS Page LIST OF FIGURES v LIST OF TABLES vi ACKNOWLEDGMENTS vii ABSTRACT OF THE THESIS viii CHAPTER 1: Introduction 1 CHAPTER 2: Literature Review 4 CHAPTER 3: Materials and Method 16 CHAPTER 4: Result 27 CHAPTER 5: Discussion 45 CHAPTER 6: Conclusion 52 REFERENCES CITED 53 APPENDIX A. 59 APPENDIX B. 61 ii" " LIST OF FIGURES Page Figure 1.1 Location of Lake Skinner and MWDSC service area 2 Figure 2.1 Structure of Microcystin-LR 5 Figure 2.2 Relationship between sensitivity and selectivity of analytical methods of Microcystin 8 Figure 2.3 ELISA standard curves for cyanobacterial cyclic peptide toxin congeners 10 Figure 2.4 Example of the ELISA assay standard curve used in this study 10 Figure 2.5 The degradative pathway of Microcystin-LR and -

BIOGEOCHEMICAL PROCESSES in ANTARCTIC AQUATIC ENVIRONMENTS: LINKAGES and LIMITATIONS by Trista Juliana Vick-Majors a Thesis Subm
BIOGEOCHEMICAL PROCESSES IN ANTARCTIC AQUATIC ENVIRONMENTS: LINKAGES AND LIMITATIONS by Trista Juliana Vick-Majors A thesis submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Ecology and Environmental Sciences MONTANA STATE UNIVERSITY Bozeman, Montana January 2016 ©COPYRIGHT by Trista Juliana Vick-Majors 2016 All Rights Reserved ii ACKNOWLEDGEMENTS I would like to thank my advisor Dr. John Priscu, for giving me the opportunity to be involved in so many amazing projects, for opening the door to the Antarctic (I didn’t even know it had one), and for giving me the freedom and support to explore my ideas. I am especially grateful to my committee members, John Dore, Eric Boyd, Anne Camper, and Dan Miller for their support and guidance. Special thanks to John Dore for seeing me through both of my graduate degrees, and Eric Boyd for always having an open door. The work in this dissertation would not have been possible without collaborations with many excellent scientists on the McMurdo LTER, the WISSARD Project, and beyond. Amanda Achberger and Alex Michaud – “we’re still in this!” Thank you for your camaraderie and talking science with me. My research and fieldwork would not have been possible without Brent Christner and Mark Skidmore, and I am grateful for their support. Special thanks to Susan Kelly, for being a wonderful friend and advocate, and giving me the opportunity to share my science with the public. Pamela Santibáñez, thank you for your constant support and sharing your wonderful mind with me. My friends and family have seen me though a long road to get here, and I am grateful to all of them. -

Proteobacteria from the Human Skin Microbiota: Species-Level Diversity and Hypotheses C
Proteobacteria from the human skin microbiota: Species-level diversity and hypotheses C. Cosseau, S. Romano-Bertrand, H. Duplan, O. Lucas, I. Ingrassia, Christel Hénocq-Pigasse, Christine Roques, E. Jumas-Bilak To cite this version: C. Cosseau, S. Romano-Bertrand, H. Duplan, O. Lucas, I. Ingrassia, et al.. Proteobacteria from the human skin microbiota: Species-level diversity and hypotheses. One Health, Elsevier, 2016, 2, pp.33-41. 10.1016/j.onehlt.2016.02.002. hal-01813975 HAL Id: hal-01813975 https://hal.archives-ouvertes.fr/hal-01813975 Submitted on 9 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Open Archive Toulouse Archive Ouverte OATAO is an open access repository that collects the work of Toulouse researchers and makes it freely available over the web where possible This is a publisher’s version published in: http://oatao.univ-toulouse.fr/20496 Official URL: https://doi.org/10.1016/j.onehlt.2016.02.002 To cite this version: Cosseau, Céline and Romano, Sara and Duplan, Hélène and Lucas, O. and Ingrassia, Isabelle and Hénocq-Pigasse, Christel and Roques, Christine and Bilak, Estelle Proteobacteria from the human skin microbiota: Species-level diversity and hypotheses. -
Glycocholate Taurocholate Taurodeoxycholic Acid
0.6 0.4 0.2 0.0 0.2 0.4 Glycocholate Taurocholate 0.6 Taurodeoxycholic acid Taurochenodeoxycholate Glycochenodeoxycholate AMP Putrescine 0.8 Biliverdin Hexose phosphate Sedoheptulose 1-phosphate Pentose phosphates O-tetradecanoyl-L-carnitine O-dodecanoyl-carnitine Tetradecenoyl Carnitine Serotonin Lactate L-Palmitoylcarnitine (15Z)-Tetracosenoic acid octenoyl-l-carnitine O-dodecenoyl-carnitine O-Decanoyl-L-carnitine O-Decenoyl-L-carnitine Citrate hexanoyl-L-carnitine L-octanoylcarnitine Succinate N-Acetylneuraminate Itaconate Fumarate Malate 6-Phospho-D-gluconate Glutathione S-Glutathionyl-L-cysteine Uracil Nicotinamide Adenosine Glutathione disulfide Cadaverine UDP-glucose Spermidine Acetylcholine Taurine Hypoxanthine Xanthine D-Ribose acyl-C5-OH Dimethylglycine acyl-C5 Pantothenol acyl-C4-OH Phosphate Hexadecenoyl-carnitine Guanosine Inosine Spermine Ethanolamine phosphate Decanoic acid (caprate) Dodecanoic acid D-Arabitol Creatine ( )12(13)-DiHOME ( )9(10)-DiHOME Ursodeoxycholic acid Taurolithocholic acid 3alpha-12alpha-Dihydroxy-5beta-cholanate Chenodeoxycholic acid propionyl-carnitine acetyl-carnitine butanoyl-l-carnitine Choline Creatinine Adenine D-Glyceraldehyde 3-phosphate/Glycerone phosphate Phosphocreatine Glycerol 3-phosphate Pyruvate 2-Oxoglutarate L-Adrenaline acyl-C4-DC Dodecanedioic acid Indole-3-acetate TMAO (trimethylamine N-oxide) Urate Cystathionine Leucine Isoleucine Ascorbate 2-Hydroxyglutarate/Citramalate Dehydroascorbate L-Citrulline L-Carnitine D-Glucose UDP-N-acetyl-D-glucosamine Bilirubin Betaine 2/3-Phospho-D-glycerate -

WEE-THESIS-2019.Pdf (1.820Mb)
MICROBIAL ABUNDANCE AND DIVERSITY IN RESPONSE TO NUTRIENT ADDITION IN SUBSEAFLOOR OCEAN CRUST AT ATLANTIS BANK, SOUTHWEST INDIAN RIDGE A Thesis by Shu Ying Wee Submitted to the Office of Graduate and Professional Studies of Texas A&M University in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE Chair of Committee, Jason B. Sylvan Committee Members, Jessica Labonté Shari Yvon-Lewis Head of Department, Shari Yvon-Lewis August 2019 Major Subject: Oceanography Copyright 2019 Shu Ying Wee ABSTRACT Olivine in the presence of seawater will undergo a reaction known as serpentinization to form the mineral serpentine in addition to hydrogen, methane, and short chain hydrocarbons, which can be metabolized by microorganisms. Environments where serpentinization reactions occur are therefore hypothesized to support microbial life. The goal of the International Ocean Discovery Program (IODP) Expedition 360 (X360) was to recover a representative transect of the lower oceanic crust formed at Atlantis Bank, an oceanic core complex on the southwest Indian Ridge. Recovered cores were primarily gabbro and olivine gabbro, which may potentially host serpentinization reactions and associated microbial life. The goal of this thesis project was to quantify in situ microbial cells, analyze the microbial community structure of the in situ rock samples, and assess if nutrient supply influences the microbial community and methane production using a nutrient addition incubation approach. It was found that the microbial cell abundance is positively correlated to vein presence in rocks for certain depths. Microbial community diversity, assessed via 16S rRNA amplicon analysis, was extremely variable with depth. Additionally, different nutrient treatments added to incubations from twelve depths did not have an observable effect on the microbial diversity or methane production. -

Microbial Biodiversity Within the Vibrionaceae Michele K
MICROBIAL BIODIVERSITY WITHIN THE VIBRIONACEAE MICHELE K. NISHIGUCHI AND BRYAN W. JONES New Mexico State University, Department of Biology Box 30001, MSC 3AF, Las Cruces, NM, 88003-8001 USA 1. The Vibrionaceae 1.1. A GENERAL DESCRIPTION Vibrio takes its name from the Latin word Vibrare, meaning ‘to wave’. Otto Müller first used the word Vibrio as a descriptor in the 18th century to describe bacteria with an elongated shape observed in culture (Rossello-Mora and Amann 2001). The family Vibrionaceae, first described by Véron (1965), resides within the g-proteobacteria, one of the five subdivisions of the phylum Proteobacteria within the domain Bacteria. All Proteobacteria are gram negative. This extremely metabolically diverse group of bacteria of medical and industrial importance has been divided into five subdivisions (a, b, d, g, and e) on the basis of 16S rRNA sequence data. The g-Proteobacteria are divided into three major subgroups, denoted g-1, g-2, and g-3. Members of the g-1 and g-2 subgroups contain sulfur producing photosynthetic bacteria and members of the family Legionella, respectively. The g-3 subgroup consist of a “potpourri” of organisms, including oceanospirilla, many pseudomonads, the enterics, and the Vibrionaceae (Woese et al. 1985). Vibrionaceae is one of 22 families within the 14 orders of the g-Proteobacteria. Members of this family were first described as oxidase-positive, motile bacteria with polar flagella (Krieg and Holt 1984). More recent data demonstrate that all Vibrionaceae species are either straight or curved rods, with some species not having oxidase-positive phenotypes (Vibrio metschnikovii, V.