Resistance to Juvenile Hormone and an Insect Growth Regulator In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Global Insecticide Use for Vector-Borne Disease Control
WHO/CDS/NTD/WHOPES/GCDPP/2007.2 GLOBAL INSECTICIDE USE FOR VECTOR-BORNE DISEASE CONTROL M. Zaim & P. Jambulingam DEPARTMENT OF CONTROL OF NEGLECTED TROPICAL DISEASES (NTD) WHO PESTICIDE EVALUATION SCHEME (WHOPES) First edition, 2002 Second edition, 2004 Third edition, 2007 © World Health Organization 2007 All rights reserved. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. All reasonable precautions have been taken by the World Health Organization to verify the information contained in this publication. However, the published material is being distributed without warranty of any kind, either express or implied. The responsibility for the interpretation and use of the material lies with the reader. In no event shall the World Health Organization be liable for damages arising from its use. The named authors alone are responsible for the views expressed in this publication. CONTENTS Page Acknowledgements i Introduction 1 Collection of information 2 Data analysis and observations on reporting 3 All uses in vector control 6 Malaria vector control 22 Dengue vector control 38 Chagas disease vector control 48 Leishmaniasis vector control 52 Other vector-borne disease control 56 Selected insecticides – DDT 58 Selected insecticides – Insect growth regulators 60 Selected insecticides – Bacterial larvicides 62 Country examples 64 Annex 1. -
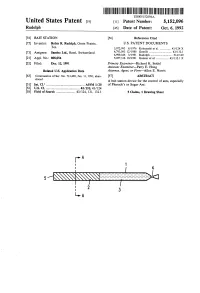
United States Patent (19) 11 Patent Number: 5,152,096 Rudolph 45 Date of Patent: Oct
III USOO5152096A United States Patent (19) 11 Patent Number: 5,152,096 Rudolph 45 Date of Patent: Oct. 6, 1992 (54) BAIT STATION (56) References Cited (75) Inventor: Robin R. Rudolph, Grain Prairie, U.S. PATENT DOCUMENTS Tex. 3,972,993 8/1976 Kobayashi et al................ 43/124 X 4,793,093 12/1988 Gentile ............................... 43/132.1 (73) Assignee: Sandoz Ltd., Basel, Switzerland 4,999,346 3/1991 Rudolph .............................. 514/120 21 Appl. No.: 808,054 5,057,316 10/1991 Gunner et al. ................. 43/132.1 X 22 Filed: Dec. 12, 1991 Primary Examiner-Richard K. Seidel Assistant Examiner-Patty E. Hong Related U.S. Application Data Attorney, Agent, or Firm-Allen E. Norris 63 Continuation of Ser. No. 713,480, Jun. 11, 1991, aban 57) ABSTRACT doned. A bait station device for the control of ants, especially (51) Int. Cl. ............................................... A0M 1/20 of Pharaoh's or Sugar Ant. (52 U.S. C. ......................................... 43/131; 43/124 58) Field of Search ....................... 43/124, 131, 132.1 5 Claims, 1 Drawing Sheet U.S. Patent Oct. 6, 1992 5,152,096 DSN a- 5,152,096 1. 2 enting the ants with a combination of an insect growth BAIT STATION regulant (IGR) bait and insecticide bait in such a way that the worker ants have to forage their way through This is a continuation of application Ser. No. the IGR bait to reach the insecticide bait. 07/713,480, filed Jun. 11, 1991, now abandoned. 5 In this way foraging worker ants will transport back The present invention concerns a bait station device to nests for feeding of the colony IGR bait and upon for the control of ants, especially of Pharaoh's or Sugar exhausting the available IGR bait will themselves ingest Ant. -

Historical Perspectives on Apple Production: Fruit Tree Pest Management, Regulation and New Insecticidal Chemistries
Historical Perspectives on Apple Production: Fruit Tree Pest Management, Regulation and New Insecticidal Chemistries. Peter Jentsch Extension Associate Department of Entomology Cornell University's Hudson Valley Lab 3357 Rt. 9W; PO box 727 Highland, NY 12528 email: [email protected] Phone 845-691-7151 Mobile: 845-417-7465 http://www.nysaes.cornell.edu/ent/faculty/jentsch/ 2 Historical Perspectives on Fruit Production: Fruit Tree Pest Management, Regulation and New Chemistries. by Peter Jentsch I. Historical Use of Pesticides in Apple Production Overview of Apple Production and Pest Management Prior to 1940 Synthetic Pesticide Development and Use II. Influences Changing the Pest Management Profile in Apple Production Chemical Residues in Early Insect Management Historical Chemical Regulation Recent Regulation Developments Changing Pest Management Food Quality Protection Act of 1996 The Science Behind The Methodology Pesticide Revisions – Requirements For New Registrations III. Resistance of Insect Pests to Insecticides Resistance Pest Management Strategies IV. Reduced Risk Chemistries: New Modes of Action and the Insecticide Treadmill Fermentation Microbial Products Bt’s, Abamectins, Spinosads Juvenile Hormone Analogs Formamidines, Juvenile Hormone Analogs And Mimics Insect Growth Regulators Azadirachtin, Thiadiazine Neonicotinyls Major Reduced Risk Materials: Carboxamides, Carboxylic Acid Esters, Granulosis Viruses, Diphenyloxazolines, Insecticidal Soaps, Benzoyl Urea Growth Regulators, Tetronic Acids, Oxadiazenes , Particle Films, Phenoxypyrazoles, Pyridazinones, Spinosads, Tetrazines , Organotins, Quinolines. 3 I Historical Use of Pesticides in Apple Production Overview of Apple Production and Pest Management Prior to 1940 The apple has a rather ominous origin. Its inception is framed in the biblical text regarding the genesis of mankind. The backdrop appears to be the turbulent setting of what many scholars believe to be present day Iraq. -

Evaluation of Indoxacarb and Fipronil (S)-Methoprene Topical Spot-On
Dryden et al. Parasites & Vectors 2013, 6:366 http://www.parasitesandvectors.com/content/6/1/366 RESEARCH Open Access Evaluation of indoxacarb and fipronil (s)-methoprene topical spot-on formulations to control flea populations in naturally infested dogs and cats in private residences in Tampa FL. USA Michael W Dryden1*, Patricia A Payne1, Vicki Smith1, Monica Chwala1, Emery Jones1, Jacob Davenport1, Gabrielle Fadl1, Maria F Martinez-Perez de Zeiders1, Kathleen Heaney2, Pamela Ford2 and Fangshi Sun2 Abstract Background: A study was conducted to evaluate and compare the effectiveness of two different spot-on topical flea products to control flea infestations on naturally infested dogs and cats in Tampa, FL USA. Methods: Thirty-two dogs and 3 cats with natural flea infestations living in 18 homes were treated topically with a 19.53% w/w spot-on formulation of indoxacarb. Another thirty dogs and 2 cats living in 19 different homes were treated topically with either fipronil (9.8% w/w)/(s)-methoprene (8.89% w/w) or fipronil (9.8% w/w)/(s)-methoprene (11.8% w/w), respectively. All products were applied according to label directions by study investigators on day 0 and again between days 28 and 30. Flea populations on pets were assessed using visual area counts and premise flea infestations were assessed using intermittent-light flea traps on days 0, 7, 14, 21, 28–30, 40–45, and 54–60. Results: A single application of the indoxacarb or fipronil (s)-methoprene formulations reduced flea populations on pets by 97.8% and 85.5%, respectively within 7 days. -

Juvenile Hormone Affects the Development and Strength of Circadian
bioRxiv preprint doi: https://doi.org/10.1101/2020.05.24.101915; this version posted May 25, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Juvenile hormone affects the development and strength of circadian rhythms in young bumble bee (Bombus terrestris) workers Atul Pandey1, Uzi Motro1,2, Guy Bloch1,2* 1) Department of Ecology, Evolution, and Behavior, The Hebrew University of Jerusalem, Jerusalem, Israel 2) The Federmann Center for the Study of Rationality, The Hebrew University of Jerusalem, Jerusalem, Israel *Corresponding author Guy Bloch: [email protected], Phone: +972-26584320 Short title: The juvenile hormone affects circadian rhythms in a bumble bee Keywords: (5 minimum) Hormone, Juvenile hormone; bumble bee, Bombus terrestris; circadian rhythms; locomotor activity; sleep, gonadotropin bioRxiv preprint doi: https://doi.org/10.1101/2020.05.24.101915; this version posted May 25, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract The circadian and endocrine systems influence many physiological processes in animals, but little is known on the ways they interact in insects. We tested the hypothesis that juvenile hormone (JH) influences circadian rhythms in the social bumble bee Bombus terrestris. JH is the major gonadotropin in this species coordinating processes such as vitellogenesis, oogenesis, wax production, and behaviors associated with reproduction. It is unknown however, whether it also influences circadian processes. We topically treated newly-emerged bees with the allatoxin Precocene-I (P-I) to reduce circulating JH titers and applied the natural JH (JH-III) for replacement therapy. -

Juvenile Hormone Esterases of Lepidoptera I
Journal J Comp Physiol (1982) 148:1-10 of Comparative Physiology, B Springer-Verlag 1982 Juvenile Hormone Esterases of Lepidoptera I. Activity in the Hemolymph During the Last Larval Instar of 1 ! Species Davy Jones, Grace Jones, Keith D. Wing, Maria Rudnicka*, and Bruce D. Hammock Departments of Entomology and Environmental Toxicology, University of California, Davis, California 95616, USA Accepted May 1, 1982 Summary. The juvenile hormone esterase (JHE) ined and has been suggested as a mode of JH titer titer was measured during the last larval instar of regulation necessary for normal development (Gil- 11 species of Lepidoptera (Pier& rapae, Junonia bert et al. 1978). In fact, recent data are consistent coenia, Danaus plexippus, Hernileuca nevadensis, with a model in which JH biosynthesis by the cor- Pectinophora gossypiella, Spodoptera exigua, Or- pora allata is reduced but not halted, and the low gyia vetusta, Ephestia elutella, Galleria mellonella, JH titers necessary for prothoracicotropic hor- Manduca sexta and Estigmene acrea). All species mone effects result from increased JH hydrolysis had a peak of JHE at or near the time of wander- (Sparks and Hammock 1980). The first lepidop- ing. The peak activity at this time ranged from teran titered during the last larval instar for ihemo- 0.8 to 388 nmoles JH III cleaved/min-ml. All spe- lymph JH esterase (JHE) activity (Manduca sexta cies except J. coenia had a second peak of JHE L.) possessed two peaks of JHE, each correlated during the late prepupal stage. The height of the with an abrupt decline in hemolymph JH (Weirich second peak ranged from 0.4 to 98.4 nmoles/min. -

Juvenile Hormone Regulation of Drosophila Aging Rochele Yamamoto Brown University
Ecology, Evolution and Organismal Biology Ecology, Evolution and Organismal Biology Publications 2013 Juvenile hormone regulation of Drosophila aging Rochele Yamamoto Brown University Hua Bai Brown University Adam G. Dolezal Iowa State University, [email protected] Gro Amdam Arizona State University Marc Tatar Brown University Follow this and additional works at: http://lib.dr.iastate.edu/eeob_ag_pubs Part of the Cell and Developmental Biology Commons, Ecology and Evolutionary Biology Commons, Entomology Commons, and the Genetics Commons The ompc lete bibliographic information for this item can be found at http://lib.dr.iastate.edu/ eeob_ag_pubs/206. For information on how to cite this item, please visit http://lib.dr.iastate.edu/ howtocite.html. This Article is brought to you for free and open access by the Ecology, Evolution and Organismal Biology at Iowa State University Digital Repository. It has been accepted for inclusion in Ecology, Evolution and Organismal Biology Publications by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Yamamoto et al. BMC Biology 2013, 11:85 http://www.biomedcentral.com/1741-7007/11/85 RESEARCH ARTICLE Open Access Juvenile hormone regulation of Drosophila aging Rochele Yamamoto1, Hua Bai1, Adam G Dolezal2,3, Gro Amdam2 and Marc Tatar1* Abstract Background: Juvenile hormone (JH) has been demonstrated to control adult lifespan in a number of non-model insects where surgical removal of the corpora allata eliminates the hormone’s source. In contrast, little is known about how juvenile hormone affects adult Drosophila melanogaster. Previous work suggests that insulin signaling may modulate Drosophila aging in part through its impact on juvenile hormone titer, but no data yet address whether reduction of juvenile hormone is sufficient to control Drosophila life span. -

Geographical Distribution and Selection of European Honey Bees Resistant to Varroa Destructor
insects Review Geographical Distribution and Selection of European Honey Bees Resistant to Varroa destructor Yves Le Conte 1,* , Marina D. Meixner 2, Annely Brandt 2, Norman L. Carreck 3,4 , Cecilia Costa 5, Fanny Mondet 1 and Ralph Büchler 2 1 INRAE, Abeilles et Environnement, 84914 Avignon, France; [email protected] 2 Landesbetrieb Landwirtschaft Hessen, Bee Institute, Erlenstrasse 9, 35274 Kirchhain, Germany; [email protected] (M.D.M.); [email protected] (A.B.); [email protected] (R.B.) 3 Carreck Consultancy Ltd., Woodside Cottage, Dragons Lane, Shipley RH13 8GD, West Sussex, UK; [email protected] 4 Laboratory of Apiculture and Social Insects, University of Sussex, Falmer, Brighton BN1 9QG, East Sussex, UK 5 CREA Research Centre for Agriculture and Environment, via di Saliceto 80, 40128 Bologna, Italy; [email protected] * Correspondence: [email protected] Received: 15 October 2020; Accepted: 3 December 2020; Published: 8 December 2020 Simple Summary: The parasitic mite Varroa destructor is a major challenge to honey bee populations worldwide. Some honey bee populations are resistant to the mite, but most of the commercially used stocks are not and rely on chemical treatment. In this article, we describe known varroa-resistant populations and the mechanisms which have been identified as responsible for survival of colonies without beekeeper intervention to control the mite. We review traits that have potential in breeding programs, discuss the role played by V. destructor as a vector for virus infections, and the changes in mite and virus virulence which could play a role in colony resistance. -

Pests of the Flower Garden Phillip E
Pests of the Flower Garden Phillip E. Sloderbeck Entomologist Southwest Area Office This publication is meant to help ent names. One of the more popular prey, predators and parasites. It is im- gardeners select insecticides for use groups of insecticides labeled for portant to select and use insecticides in flower gardens. It lists some of the home use are the pyrethroids, which carefully. common pests associated with flow- come in a variety of names such as When selecting insecticides, buy in ers and some of the active ingredients bifenthrin, cyfluthrin, permethrin and quantities that can be used in a reason- found in insecticides labeled for use esefenvalerate. Many of these com- able amount of time. Look for prod- on ornamental plants. The list contains pounds end in “-thrin,” but not all. ucts that can be used for more than common active ingredients for each Many have a broad spectrum, but the one pest. For example, if a gardener pest from the Kansas pesticide data- lists of pests controlled by each pyre- has problems with aphids and mealy- base. Other effective materials may throid varies. bugs, it might be best to buy a product also be available. Gardeners should Remember that to be a pest, insects that controls both rather than buying check labels carefully and visit local have to be present in substantial num- separate products for each pest. Re- retail outlets to determine which prod- bers. Spotting one or two insects in a member that if it is necessary to treat ucts are best suited for a particular garden should not trigger an insecti- pests several times during the season, pest problem. -

Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2019 Theinternational Programme on Chemical Safety (IPCS) Was Established in 1980
The WHO Recommended Classi cation of Pesticides by Hazard and Guidelines to Classi cation 2019 cation Hazard of Pesticides by and Guidelines to Classi The WHO Recommended Classi The WHO Recommended Classi cation of Pesticides by Hazard and Guidelines to Classi cation 2019 The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2019 TheInternational Programme on Chemical Safety (IPCS) was established in 1980. The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management of chemicals. This publication was developed in the IOMC context. The contents do not necessarily reflect the views or stated policies of individual IOMC Participating Organizations. The Inter-Organization Programme for the Sound Management of Chemicals (IOMC) was established in 1995 following recommendations made by the 1992 UN Conference on Environment and Development to strengthen cooperation and increase international coordination in the field of chemical safety. The Participating Organizations are: FAO, ILO, UNDP, UNEP, UNIDO, UNITAR, WHO, World Bank and OECD. The purpose of the IOMC is to promote coordination of the policies and activities pursued by the Participating Organizations, jointly or separately, to achieve the sound management of chemicals in relation to human health and the environment. WHO recommended classification of pesticides by hazard and guidelines to classification, 2019 edition ISBN 978-92-4-000566-2 (electronic version) ISBN 978-92-4-000567-9 (print version) ISSN 1684-1042 © World Health Organization 2020 Some rights reserved. -

BACWA Methoprene Comment Letter
December 22, 2020 Cody Kendrick Office of Pesticide Programs (OPP) Regulatory Public Docket Center (28221T) U.S. Environmental Protection Agency (EPA) 1200 Pennsylvania Ave., NW Washington, DC 20460–0001 Subject: Methoprene – Combined Final Work Plan and Proposed Interim Registration Review Decision (EPA–HQ–OPP–2013–0586) Dear Mr. Kendrick: On behalf of the Bay Area Clean Water Agencies (BACWA), we thank you for the opportunity to comment on the Combined Final Work Plan and Proposed Interim Registration Review Decision (PID) for methoprene. BACWA’s members include 55 publicly owned wastewater treatment facilities (“POTWs”) and collection system agencies serving 7.1 million San Francisco Bay Area residents. We take our responsibilities for safeguarding receiving waters seriously. BACWA is especially interested in pesticides that are used in manners that have transport pathways to the sanitary sewer, as even the most sophisticated wastewater treatment plants cannot fully remove complex chemicals like pesticides. BACWA has a strong interest in methoprene due to use in pet flea control and abandoned swimming pool products. The purposes of this letter are: (1) to request that the qualitative discussion of sewer discharges in the Proposed Interim Decision be replaced with a quantitative (i.e., modeled) down-the-drain evaluation addressing discharges of methoprene to the sewer due to companion animal treatments and (2) to request that the PID require registrants to include the same swimming discharge instructions on methoprene products that are being required by EPA on all other pool products (e.g., lithium hypochlorite, copper, chlorine, halohydantoins, terbuthylazine, inorganic halides, zinc salts, boric acid/sodium salts) as suggested by BPPD’s own risk assessors.1 1 Jones, R.S., Risk Assessment Branch, Biopesticides and Pollution Prevention Division (2020). -

Managing Pesticide Poisoning Risk and Understanding the Signs and Symptoms Clyde L
EC2505 Revised June 2018 Managing Pesticide Poisoning Risk and Understanding the Signs and Symptoms Clyde L. Ogg, Extension Educator Jan R. Hygnstrom, Project Manager Cheryl A. Alberts, Project Coordinator Erin C. Bauer, Entomology Lecturer The potential for accidents with pesticides is real. Ac- cidental exposure or overexposure to pesticides can have seri- ous consequences. While most pesticides can be used with relatively little risk when label directions are followed, some are extremely toxic and require special precautions. The Poison Control Centers receive about 90,000 calls each year related to pesticide exposures. Pesticides are re- sponsible for about 3 percent of all accidental exposures to children 5 years and younger and about 4 percent for adults. In addition, pesticides are the cause of about 3 percent of children’s deaths reported to the Poison Control Centers. Routes of Exposure Pesticides can enter the human body three ways: 1) der- mal exposure, by absorption through the skin or eyes; 2) oral exposure, through the mouth; and 3) through inhalation or respiratory exposure, by inhaling into the lungs. Some classify exposure through the eyes as ocular exposure. Dermal exposure results in absorption immediately after Figure 1. Absorption rates of different a pesticide contacts the skin or eyes. Absorption will contin- parts of the body based on the absorption ue as long as the pesticide remains in contact with the skin or of parathion into the forearm over 24 eyes. The rate at which dermal absorption occurs is different hours. for each part of the body (Figure 1). Maiback and Feldman (1974) measured the amount of the pesticide parathion absorbed by different parts of the human body over 24 hours.