A Protocol for Use of Shortnose and Atlantic Sturgeons
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

First Verified Occurrence of the Shortnose Sturgeon (Acipenser Brevirostrum) in the James River, Virginia
19 6 Abstract—The shortnose sturgeon (Acipenser brevirostrum) is an en- First verified occurrence of the shortnose dangered species of fish that inhab- sturgeon (Acipenser brevirostrum) its the continental slope of the At- lantic Ocean from New Brunswick, in the James River, Virginia Canada, to Florida. This species has not been documented previously in the freshwater portion of any river Matthew Balazik of the Chesapeake Bay, except in the Potomac River. On 13 March 2016, a Email address for author: [email protected] shortnose sturgeon was captured in the freshwater portion of the James Center for Environmental Studies River at river kilometer 48. The Virginia Commonwealth University fish had a fork length of about 75 1000 West Cary Street cm and was likely mature. Genetic Richmond, Virginia 23284 analysis confirmed the fish was a shortnose sturgeon and was assigned to the Chesapeake Bay–Delaware population segment. Regardless of whether this shortnose sturgeon was part of a remnant Chesapeake The shortnose sturgeon (Acipenser ture of shortnose sturgeon, as well Bay population or whether its cap- brevirostrum) is an amphidromous (Spells1; Welsh et al., 2002; Mangold ture there is an indicator of an ex- sturgeon reported to inhabit the con- et al.2). Only one genetically verified pansion of range from the Delaware tinental slope of the Atlantic Ocean shortnose sturgeon was collected in River by way of the Chesapeake and from New Brunswick, Canada, to Virginia waters as part of these pro- Delaware Canal, dedicated research Florida (Gruchy and Parker, 1980; grams (Spells1; Welsh et al., 2002). is needed to determine the status of Dadswell et al., 1984; Kynard, 1997). -

Atlantic Sturgeon
Species Profile: Atlantic Sturgeon Benchmark Assessment Indicates Signs of a Slow Recovery Species Snapshot Though Challenges Towards Sustainability Remain Introduction Atlantic Sturgeon For almost 30 years, the 15 Atlantic coastal states have worked together to effectively Acipenser oxyrhynchus oxyrhynchus manage Atlantic sturgeon throughout its range from Maine to Florida. Recognizing both the importance of this ancient species and the dire status of the population, the states Management Unit: Maine to Florida implemented a 40-year coastwide moratorium on harvest through Amendment I to the Interesting Facts Atlantic Sturgeon FMP in 1998 with the goal of restoring the population of this once thriving • The species name 'oxyrhynchus' means sharp fishery. Since then, the states have invested considerable resources to research the species’ snout. biology and life history. Despite the strong conservation efforts taken, Atlantic sturgeon was added to the Endangered Species List in February 2012. A coastwide benchmark stock • Sturgeon were a key food source for U.S. settlers assessment completed by the Commission in the fall of 2017 concluded that the population along the Atlantic coast. remains depleted at the coastwide and distinct population segment (DPS) levels relative • In the late 1800s, fishing for sturgeon eggs (to to historic abundance, although the population appears to be recovering slowly since sell as caviar) attracted so many people, the trend implementation of the 1998 moratorium. was referred to as the “Black Gold Rush.” • Atlantic sturgeon are river-specific, returning to Life History their natal rivers to spawn. Atlantic sturgeon (Acipenser oxyrhynchus oxyrhynchus) are ancient fish, dating back to at least • Rather than having true scales, Atlantic sturgeon the late Cretaceous Period (66-100 million years ago). -

Pan-European Action Plan for Sturgeons
Strasbourg, 30 November 2018 T-PVS/Inf(2018)6 [Inf06e_2018.docx] CONVENTION ON THE CONSERVATION OF EUROPEAN WILDLIFE AND NATURAL HABITATS Standing Committee 38th meeting Strasbourg, 27-30 November 2018 PAN-EUROPEAN ACTION PLAN FOR STURGEONS Document prepared by the World Sturgeon Conservation Society and WWF This document will not be distributed at the meeting. Please bring this copy. Ce document ne sera plus distribué en réunion. Prière de vous munir de cet exemplaire. T-PVS/Inf(2018)6 - 2 - Pan-European Action Plan for Sturgeons Multi Species Action Plan for the: Russian sturgeon complex (Acipenser gueldenstaedtii, A. persicus-colchicus), Adriatic sturgeon (Acipenser naccarii), Ship sturgeon (Acipenser nudiventris), Atlantic/Baltic sturgeon, (Acipenser oxyrinchus), Sterlet (Acipenser ruthenus), Stellate sturgeon (Acipenser stellatus), European/Common sturgeon (Acipenser sturio), and Beluga (Huso huso). Geographical Scope: European Union and neighbouring countries with shared basins such as the Black Sea, Mediterranean, North Eastern Atlantic Ocean, North Sea and Baltic Sea Intended Lifespan of Plan: 2019 – 2029 Russian Sturgeon Adriatic Sturgeon Ship Sturgeon Atlantic or Baltic complex Sturgeon Sterlet Stellate Sturgeon Beluga European/Common Sturgeon © M. Roggo f. A. sturio; © Thomas Friedrich for all others Supported by - 3 - T-PVS/Inf(2018)6 GEOGRAPHICAL SCOPE: The Action Plan in general addresses the entire Bern Convention scope (51 Contracting Parties, including the European Union) and in particular the countries with shared sturgeon waters in Europe. As such, it focuses primarily on the sea basins in Europe: Black Sea, Mediterranean, North-East Atlantic, North Sea, Baltic Sea, and the main rivers with relevant current or historic sturgeon populations (see Table 2). -
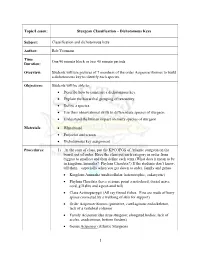
Sturgeon Classification – Dichotomous Keys Subject
Topic/Lesson: Sturgeon Classification – Dichotomous Keys Subject: Classification and dichotomous keys Author: Rob Yeomans Time One 90 minute block or two 45 minute periods Duration: Overview: Students will use pictures of 7 members of the order Acipenseriformes to build a dichotomous key to identify each species. Objectives: Students will be able to: • Describe how to construct a dichotomous key. • Explain the hierarchal grouping of taxonomy. • Define a species. • Use their observational skills to differentiate species of sturgeon. • Understand the human impact on many species of sturgeon. Materials: • Whiteboard • Projector and screen • Dichotomous key assignment Procedures: 1) At the start of class, put the KPCOFGS of Atlantic sturgeon on the board, out of order. Have the class put each category in order from biggest to smallest and then define each term (What does it mean to be in kingdom Animalia? Phylum Chordata?) If the students don’t know, tell them—especially when you get down to order, family and genus. • Kingdom Animalia (multicellular, heterotrophic, eukaryotic) • Phylum Chordata (have at some point a notochord, dorsal nerve cord, gill slits and a post-anal tail) • Class Actinopterygii (All ray finned fishes. Fins are made of bony spines connected by a webbing of skin for support) • Order Acipenseriformes (primitive, cartilaginous endoskeleton, lack of a vertebral column) • Family Acipenseridae (true sturgeon; elongated bodies, lack of scales, anadromous, bottom feeders) • Genus Acipenser (Atlantic Sturgeon) 1 • Species oxyrinchus Comment to the class that there are actually two subspecies of oxyrinchus. Acipenser oxyrinchus oxyrinchus is the Atlantic sturgeon and Acipenser oxyrinchus desotoi is the Gulf sturgeon 2) Reinforce the fact that sturgeon are a primitive fish and fossils have been found dating back 144-65 million years ago. -

The Key Threats to Sturgeons and Measures for Their Protection in the Lower Danube Region
THE KEY THREATS TO STURGEONS AND MEASURES FOR THEIR PROTECTION IN THE LOWER DANUBE REGION MIRJANA LENHARDT* Institute for Biological Research, Serbia IVAN JARIĆ, GORČIN CVIJANOVIĆ AND MARIJA SMEDEREVAC-LALIĆ Center for Multidisciplinary Studies, Serbia Abstract The six native sturgeon species have been commercially harvested in the Danube Basin for more than 2,000 years, with rapid decrease in catch by mid 19th century. Addi- tional negative effect on sturgeon populations in the Danube River was river regulation in Djerdap region, due to navigation in the late 19th century, as well as dam construction in the second half of 20th century that blocked sturgeon spawning migrations. Beside over- fishing and habitat loss, illegal trade, life history characteristics of sturgeon, lack of effective management (due to lack of transboundary cooperation and change in political situa- tion in Lower Danube Region countries) and pollution all pose serious threats on sturgeon populations in Lower Danube Region. International measures established by the Conven- tion on International Trade in Endangered Species (CITES) in late 20th century, listing of beluga (Huso huso) as an endangered species under the U.S. Endangered Species Act, as well as development of Action plan for conservation of sturgeons in the Danube River Basin, had significant impact on activities related to sturgeon protection at beginning of 21st century. These actions were aimed towards diminishment of pressure on natural sturgeon populations and aquaculture development in countries of Lower Danube Region. The main goal of the Action Plan was to raise public awareness and to create a common framework for implementation of urgent measures. -

Atlantic Sturgeon Acipenser Oxyrinchus
COSEWIC Assessment and Status Report on the Atlantic Sturgeon Acipenser oxyrinchus St. Lawrence populations Maritimes populations in Canada THREATENED 2011 COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: COSEWIC. 2011. COSEWIC assessment and status report on the Atlantic Sturgeon Acipenser oxyrinchus in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xiii + 49 pp. (www.sararegistry.gc.ca/status/status_e.cfm). Production note: COSEWIC acknowledges Robert Campbell for writing the provisional status report on the Atlantic Sturgeon, Acipenser oxyrinchus. The contractor’s involvement with the writing of the status report ended with the acceptance of the provisional report. Any modifications to the status report during the subsequent preparation of the 6-month interim and 2-month interim status report were overseen by Dr. Eric Taylor, COSEWIC Freshwater Fishes Specialist Subcommittee Co-Chair. For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment Canada Ottawa, ON K1A 0H3 Tel.: 819-953-3215 Fax: 819-994-3684 E-mail: COSEWIC/[email protected] http://www.cosewic.gc.ca Également disponible en français sous le titre Ếvaluation et Rapport de situation du COSEPAC sur l'esturgeon noir (Acipenser oxyrinchus) au Canada. Cover illustration/photo: Atlantic Sturgeon — from Cornell University Department of Natural Resources by permission. Her Majesty the Queen in Right of Canada, 2011. Catalogue No. CW69-14/636-2011E-PDF ISBN 978-1-100-18706-8 Recycled paper COSEWIC Assessment Summary Assessment Summary – May 2011 Common name Atlantic Sturgeon - St. -

Acipenser Oxyrinchus) Ecological Risk Screening Summary
Atlantic sturgeon (Acipenser oxyrinchus) Ecological Risk Screening Summary U.S. Fish and Wildlife Service, web version – 03/29/2018 Photo: Simon Pierre Barrette. Licensed under CC BY-SA 3.0. Available: https://commons.wikimedia.org/wiki/File:Acipenser_oxyrinchus_PAQ.jpg. 1 Native Range and Status in the United States Native Range From Froese and Pauly (2014): “Western Atlantic: Hamilton River, Labrador, Newfoundland, Canada to northeastern Florida, USA. Occurs occasionally in Bermuda and French Guiana [Robins and Ray 1986]. Northern Gulf of Mexico [Smith 1997]. In Europe: Baltic Sea. Landlocked populations in Lakes Ladoga and Onega (Russia), both now extirpated. Occasionally recorded from Great Britain and North Sea in Elbe drainage [Kottelat and Freyhof 2007]. Recent research revealed that this species existed in the Baltic Sea, but is now extirpated [Ludwig et al. 2002, 2008].” 1 Status in the United States From Froese and Pauly (2014): “USA: native” From NOAA Fisheries (2016): “Historically, Atlantic sturgeon were present in approximately 38 rivers in the United States from St. Croix, ME to the Saint Johns River, FL, of which 35 rivers have been confirmed to have had a historical spawning population. Atlantic sturgeon are currently present in approximately 32 of these rivers, and spawning occurs in at least 20 of them.” GBIF Secretariat (2014) contains a record in California that is assumed to be a misidentification as it is from 1956 and not substantiated elsewhere. Means of Introductions in the United States No records of non-native Acipenser oxyrinchus introductions in the United States were found. Remarks From Froese and Pauly (2014): “Near threatened globally, but extirpated in Europe [Kottelat and Freyhof 2007]. -

Genetic Diversity of Stellate Sturgeon in the Lower Danube River: the Impact of Habitat Contraction Upon a Critically Endangered Population
water Article Genetic Diversity of Stellate Sturgeon in the Lower Danube River: The Impact of Habitat Contraction upon a Critically Endangered Population Daniela Nicoleta Holostenco 1,2 , Mitică Ciorpac 1,3,*, Elena Taflan 1, Katarina Toši´c 1,4, Marian Paraschiv 1, 1 1 1 2,5, Marian Iani ,S, tefan Hont, , Radu Suciu and Geta Rîs, noveanu * 1 Danube Delta National Institute for Research and Development, 820112 Tulcea, Romania; [email protected] (D.N.H.); elena.tafl[email protected] (E.T.); [email protected] (K.T.); [email protected] (M.P.); [email protected] (M.I.); [email protected] (S, .H.); [email protected] (R.S.) 2 Doctoral School of Ecology, Faculty of Biology, University of Bucharest, 050095 Bucharest, Romania 3 Genetic Improvement Laboratory, Research Station for Cattle Breeding Dancu, 707252 Ias, i, Romania 4 Faculty of Biology, University of Belgrade, 11000 Belgrade, Serbia 5 Department of Systems Ecology and Sustainability, Faculty of Biology, University of Bucharest, 050095 Bucharest, Romania * Correspondence: [email protected] (M.C.); [email protected] (G.R.); Tel.: +40-765442892 (M.C.); +40-727803028 (G.R.) Abstract: One of the last wild populations of the critically endangered stellate sturgeon (Acipenser stellatus) survives in the Danube River. Limited knowledge about the genetic structure, ecology, and Citation: Holostenco, D.N.; Ciorpac, evolution of this species led to poor and inconsistent management decisions with an increased risk for M.; Taflan, E.; Toši´c,K.; Paraschiv, M.; species extinction in the wild. Here we show the results of genetic structure screening of the Danube Iani, M.; Hont,,S, .; Suciu, R.; River wild population over 12 years timespan. -

Acipenser Brevirostrum
AR-405 BIOLOGICAL ASSESSMENT OF SHORTNOSE STURGEON Acipenser brevirostrum Prepared by the Shortnose Sturgeon Status Review Team for the National Marine Fisheries Service National Oceanic and Atmospheric Administration November 1, 2010 Acknowledgements i The biological review of shortnose sturgeon was conducted by a team of scientists from state and Federal natural resource agencies that manage and conduct research on shortnose sturgeon along their range of the United States east coast. This review was dependent on the expertise of this status review team and from information obtained from scientific literature and data provided by various other state and Federal agencies and individuals. In addition to the biologists who contributed to this report (noted below), the Shortnose Stugeon Status Review Team would like to acknowledge the contributions of Mary Colligan, Julie Crocker, Michael Dadswell, Kim Damon-Randall, Michael Erwin, Amanda Frick, Jeff Guyon, Robert Hoffman, Kristen Koyama, Christine Lipsky, Sarah Laporte, Sean McDermott, Steve Mierzykowski, Wesley Patrick, Pat Scida, Tim Sheehan, and Mary Tshikaya. The Status Review Team would also like to thank the peer reviewers, Dr. Mark Bain, Dr. Matthew Litvak, Dr. David Secor, and Dr. John Waldman for their helpful comments and suggestions. Finally, the SRT is indebted to Jessica Pruden who greatly assisted the team in finding the energy to finalize the review – her continued support and encouragement was invaluable. Due to some of the similarities between shortnose and Atlantic sturgeon life history strategies, this document includes text that was taken directly from the 2007 Atlantic Sturgeon Status Review Report (ASSRT 2007), with consent from the authors, to expedite the writing process. -

Yangtze Sturgeon (Acipenser Dabryanus) - Sturgeons
Pond Life - Yangtze Sturgeon (Acipenser dabryanus) - Sturgeons http://www.pond-life.me.uk/sturgeon/acipenserdabryanus.php Search Pond Life... Home Sturgeons Koi Other Fish Fish Health Ponds Plants Forums Contents Yangtze Sturgeon (Acipenser dabryanus) Home Sturgeons Acipenseriformes Sturgeon Food & Feeding Sturgeon Care Sheet Sturgeon Guide Sturgeon Species List Adriatic Sturgeon Alabama Sturgeon Amu Darya Sturgeon Amur Sturgeon Atlantic Sturgeon Beluga Sturgeon Chinese Paddlefish Chinese Sturgeon Yangtze Sturgeon (Acipenser dabryanus) photo from the website of CAFS Common Sturgeon (http://zzzy.fishinfo.cn/) Diamond Sturgeon Dwarf Sturgeon by Karen Paul Green Sturgeon Description: The Yangtze Sturgeon (Acipenser dabryanus) has 8-13 dorsal scutes, 26-39 lateral Gulf Sturgeon scutes, 9-13 ventral scutes, 44-57 dorsal fin rays and 25-36 anal fin rays. Colouration ranges from Kaluga Sturgeon dark grey to brown-grey on the back to white on the ventral side. The body is rough because it is Lake Sturgeon covered with small pointed denticles. The four barbels are located closer to the mouth than the end Paddlefish of the snout. The Yangtze Sturgeon can reach 1.3 meters in length and a weight of 16kg. Pallid Sturgeon Persian Sturgeon Sakhalin Sturgeon Ship Sturgeon Shortnose Sturgeon Shovelnose Sturgeon Siberian Sturgeon Stellate Sturgeon Sterlet Syr Darya Sturgeon White Sturgeon Yangtze Sturgeon Sturgeon Videos Koi Other Fish Fish Health Yangtze Sturgeon (Acipenser dabryanus) photo from the website of CAFS Ponds (http://zzzy.fishinfo.cn/) Plants Forums Wild Distribution: Asia; restricted to the upper and middle reaches of the Yangtze River system, Search rarely seen below the Gezhouba Dam. The Yangtze Sturgeon is a potamodromous (freshwater only) species. -

Petition to List US Populations of Lake Sturgeon (Acipenser Fulvescens)
Petition to List U.S. Populations of Lake Sturgeon (Acipenser fulvescens) as Endangered or Threatened under the Endangered Species Act May 14, 2018 NOTICE OF PETITION Submitted to U.S. Fish and Wildlife Service on May 14, 2018: Gary Frazer, USFWS Assistant Director, [email protected] Charles Traxler, Assistant Regional Director, Region 3, [email protected] Georgia Parham, Endangered Species, Region 3, [email protected] Mike Oetker, Deputy Regional Director, Region 4, [email protected] Allan Brown, Assistant Regional Director, Region 4, [email protected] Wendi Weber, Regional Director, Region 5, [email protected] Deborah Rocque, Deputy Regional Director, Region 5, [email protected] Noreen Walsh, Regional Director, Region 6, [email protected] Matt Hogan, Deputy Regional Director, Region 6, [email protected] Petitioner Center for Biological Diversity formally requests that the U.S. Fish and Wildlife Service (“USFWS”) list the lake sturgeon (Acipenser fulvescens) in the United States as a threatened species under the federal Endangered Species Act (“ESA”), 16 U.S.C. §§1531-1544. Alternatively, the Center requests that the USFWS define and list distinct population segments of lake sturgeon in the U.S. as threatened or endangered. Lake sturgeon populations in Minnesota, Lake Superior, Missouri River, Ohio River, Arkansas-White River and lower Mississippi River may warrant endangered status. Lake sturgeon populations in Lake Michigan and the upper Mississippi River basin may warrant threatened status. Lake sturgeon in the central and eastern Great Lakes (Lake Huron, Lake Erie, Lake Ontario and the St. Lawrence River basin) seem to be part of a larger population that is more widespread. -

Shortnose Sturgeon Fact Sheet
Shortnose Sturgeon Acipenser brevirostrum Description: Facts: olive-yellow to gray or bluish on the back listed as endangered, it is unlawful to kill or possess this fish milky white to dark yellow on the belly long lived, like most sturgeon 5 rows of pale bony plates, called scutes males spawn every other year, females spawn every (one on the back, two on the belly and one on each third year side) semi anadromous (migrates from salt water to spawn scutes are pale and contrast with background in fresh water) 4 barbels in front of its large underslung mouth eat sludge worms, insect larvae, plants, snails, shrimp and crayfish short blunt conical snout use barbels to locate food Size: rarely exceeds 3.5 feet and 14 pounds SHORTNOSE STURGEON CATCH TOTALS Year Total 2004 1 Range: Grand Total 1 Atlantic seaboard in North America, from the St. *shortnose sturgeon were only caught during the John’s River in New Brunswick to the St. John River years shown in Florida NJ Department of Environmental Protection Division of Fish and Wildlife Bureau of Marine Fisheries www.NJFishandWildlife.com Shortnose Sturgeon Acipenser brevirostrum Historically, the Delaware Estuary has been an important habitat for two species of sturgeon: the shortnose sturgeon, and its cousin, the Atlantic sturgeon. Atlantic sturgeon primarily live in the ocean, and migrate through the estuary to spawn in freshwater. Shortnose sturgeon spend most of their time in the brackish water of the estuary, moving upstream to fresher water to spawn. Over the duration of the Delaware River seine survey, only one sturgeon has ever been caught.